How to find the chemical modification of nedosiran?
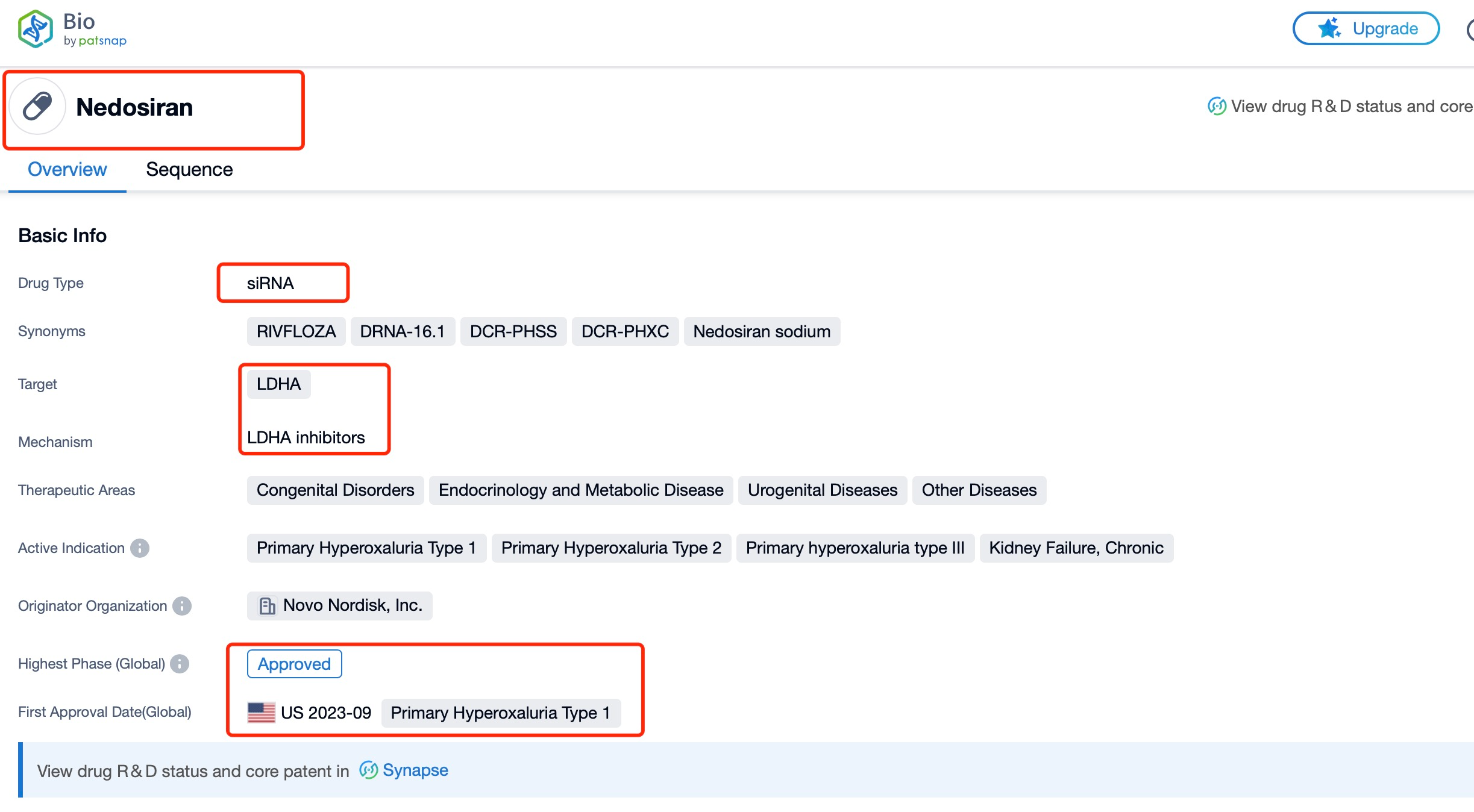
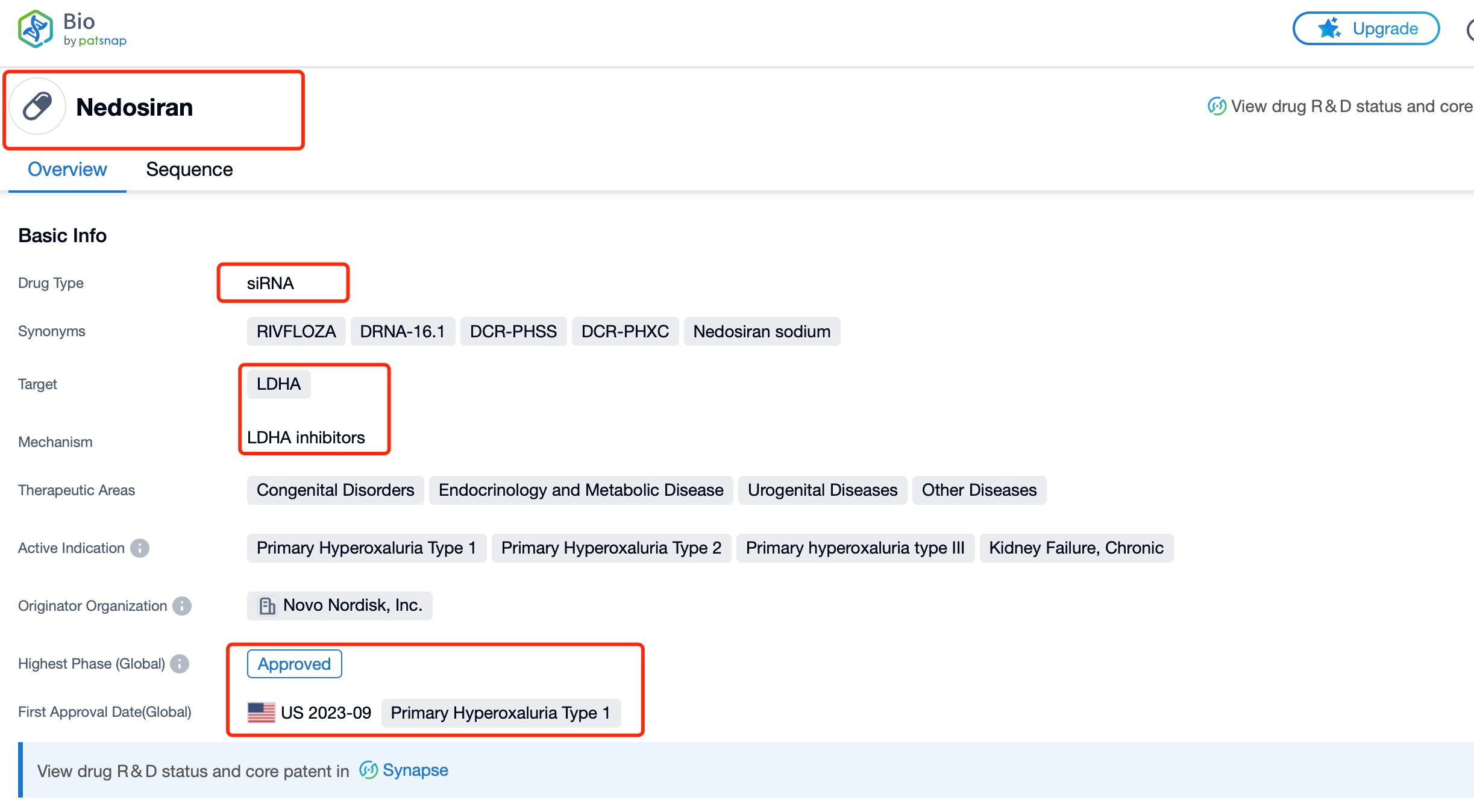
Nedosiran, also known by its brand name RIVFLOZA, is an innovative RNA interference (RNAi) therapeutic developed by Dicerna Pharmaceuticals, a subsidiary of Novo Nordisk. This groundbreaking drug targets the lactate dehydrogenase A (LDHA) gene, which plays a crucial role in the overproduction of oxalate in patients with primary hyperoxaluria (PH). Nedosiran is a small interfering RNA (siRNA) molecule designed to silence the LDHA gene, thereby reducing oxalate levels in the body. The drug is specifically indicated for the treatment of primary hyperoxaluria type 1 (PH1), a rare genetic disorder characterized by excessive oxalate production leading to kidney stones, kidney damage, and potentially life-threatening systemic oxalosis.
Summary of Research Progress of nedosiran
The research progress of nedosiran has been remarkable, showcasing its potential as a transformative therapy for PH1 patients. The drug's mechanism of action is based on the principle of RNA interference, where the siRNA molecule binds to and degrades the messenger RNA (mRNA) of the LDHA gene, effectively preventing the production of the enzyme responsible for oxalate synthesis. This targeted approach allows for a more precise and potentially more effective treatment compared to traditional therapies.
In terms of global listing, nedosiran received its first approval on September 29, 2023, from the U.S. Food and Drug Administration (FDA). This milestone marked a significant advancement in the treatment of PH1, offering a new option for patients aged 9 years and older with relatively preserved kidney function (estimated glomerular filtration rate ≥ 30 mL/min/1.73 m2). The approval was based on compelling clinical trial data demonstrating the drug's efficacy and safety profile.
The global competition pattern for PH1 treatments has evolved with the introduction of nedosiran. Prior to its approval, the landscape was dominated by supportive care measures and, in severe cases, combined liver-kidney transplantation. The arrival of RNAi therapeutics has shifted the paradigm, with nedosiran joining Lumasiran (Oxlumo) as the second FDA-approved RNAi drug for PH1. While Lumasiran targets the glycolate oxidase (GO) enzyme, nedosiran's unique approach of targeting LDHA offers a complementary strategy, potentially broadening the treatment options for PH patients.
Clinical research on nedosiran has progressed through various phases, culminating in its FDA approval. The PHYOX clinical trial program has been instrumental in demonstrating the drug's efficacy and safety. The phase 1 PHYOX1 trial (NCT03392896) showed promising results, with a mean maximal reduction of urinary oxalate levels of 66% in patients with PH1. The subsequent phase 2 PHYOX2 trial (NCT03847909) achieved its primary endpoint, demonstrating a statistically significant reduction in urinary oxalate excretion compared to placebo. These positive outcomes paved the way for the drug's approval and have set the stage for further investigations into its long-term efficacy and safety.
Sequence Information and Characteristics of nedosiran
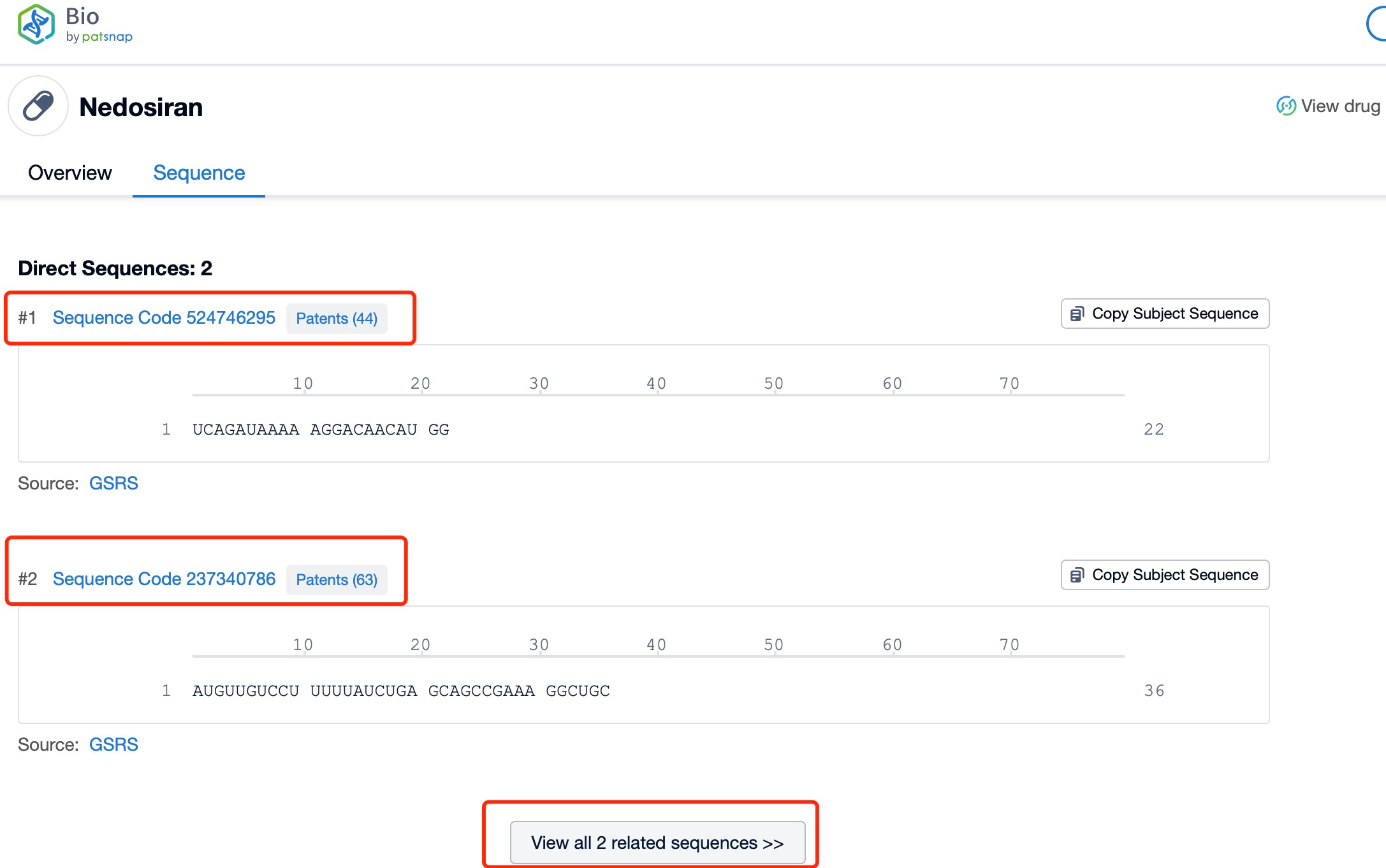
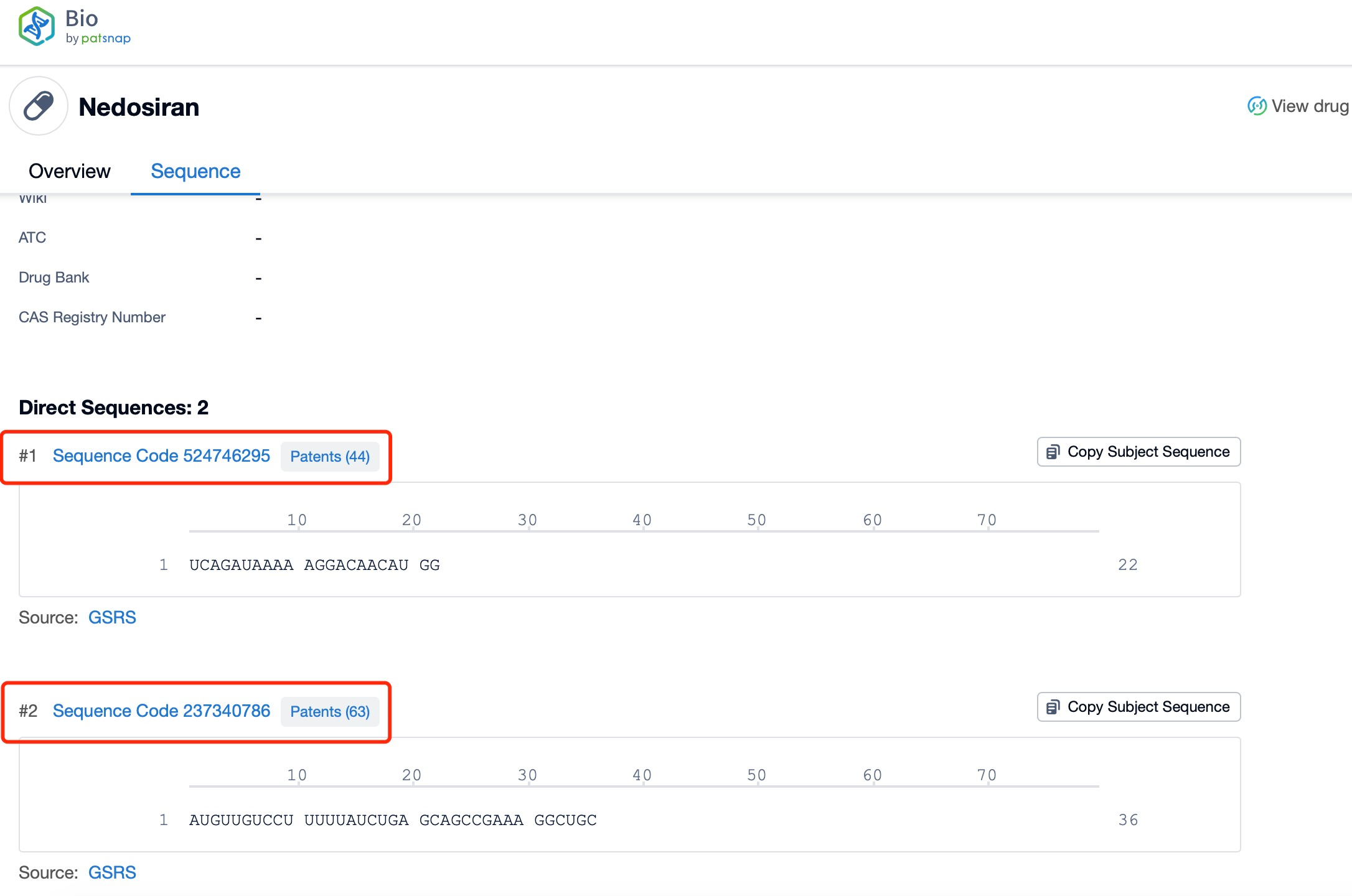
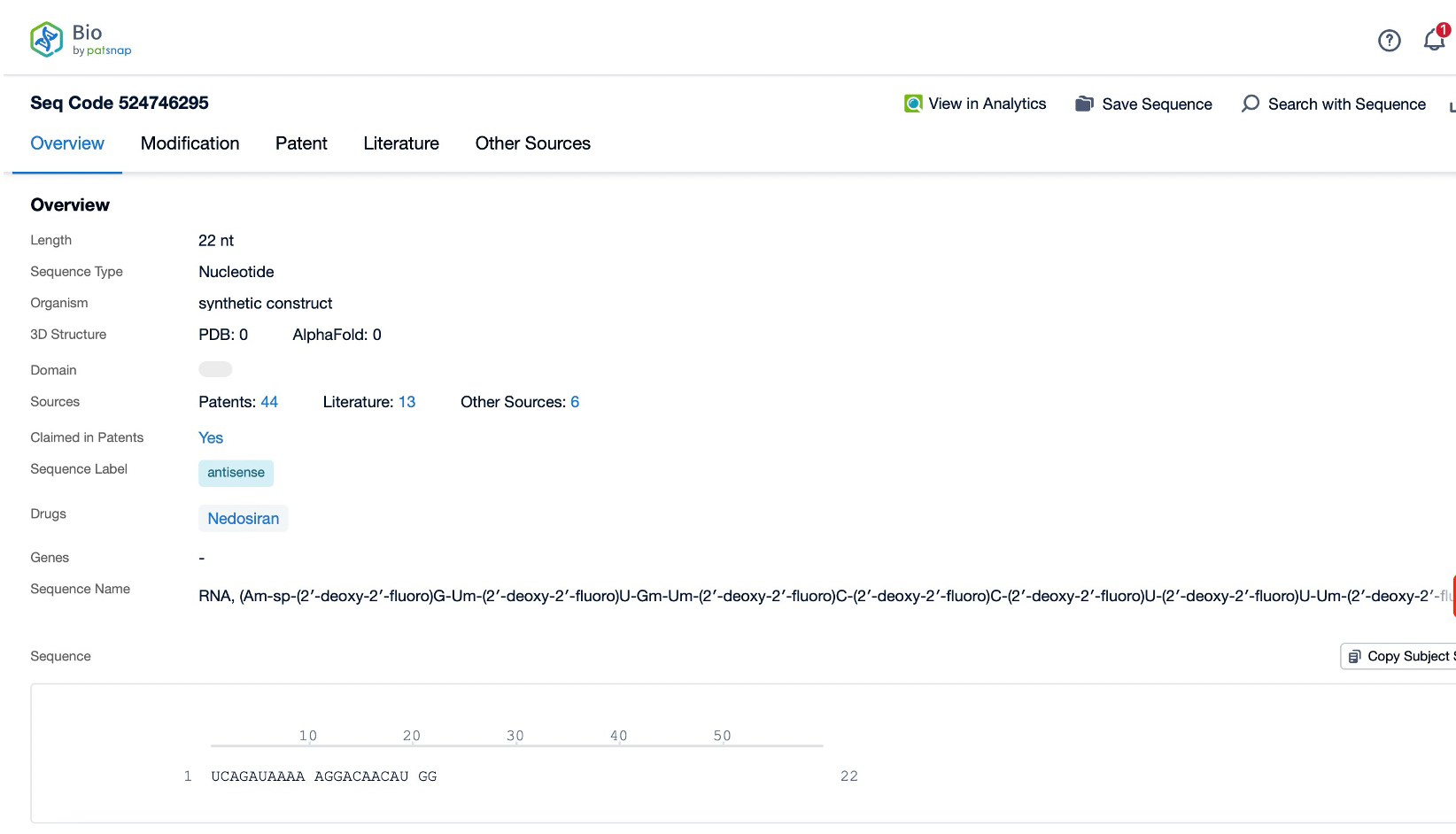
The sequence information and characteristics of nedosiran are crucial to understanding its specificity and efficacy. As an siRNA molecule, nedosiran consists of a short, double-stranded RNA sequence designed to complement the mRNA of the LDHA gene. While the exact nucleotide sequence is proprietary, the general structure of siRNA molecules typically includes a sense (passenger) strand and an antisense (guide) strand, nedosiran approximately 22-36 nucleotides in length. The antisense strand is designed to be perfectly complementary to the target LDHA mRNA sequence, ensuring high specificity and potency in gene silencing.
One of the key characteristics of nedosiran is its stability and ability to maintain efficacy over time. This is achieved through chemical modifications to the RNA backbone and sugar moieties, which enhance resistance to nuclease degradation and improve pharmacokinetic properties. These modifications are carefully balanced to maintain the molecule's ability to interact with the RNA-induced silencing complex (RISC) while protecting it from rapid degradation in the biological environment.
The design of nedosiran also takes into account the need for efficient cellular uptake and tissue-specific targeting. To this end, the drug is likely conjugated to N-acetylgalactosamine (GalNAc), a ligand that binds to asialoglycoprotein receptors on hepatocytes. This conjugation strategy enhances liver-specific delivery, which is crucial for targeting the hepatic production of oxalate in PH1 patients.
Chemical Modification and Action of nedosiran
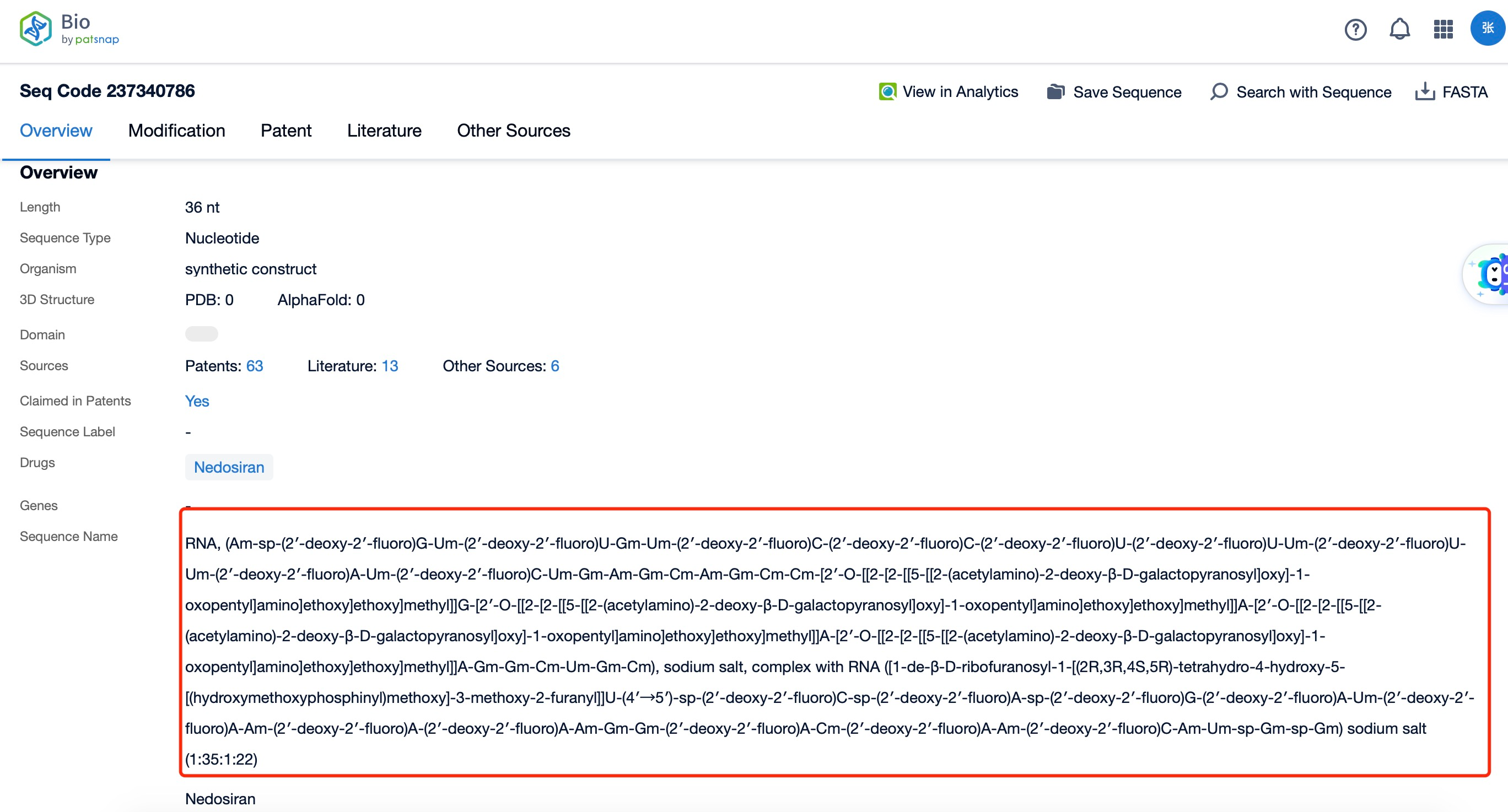
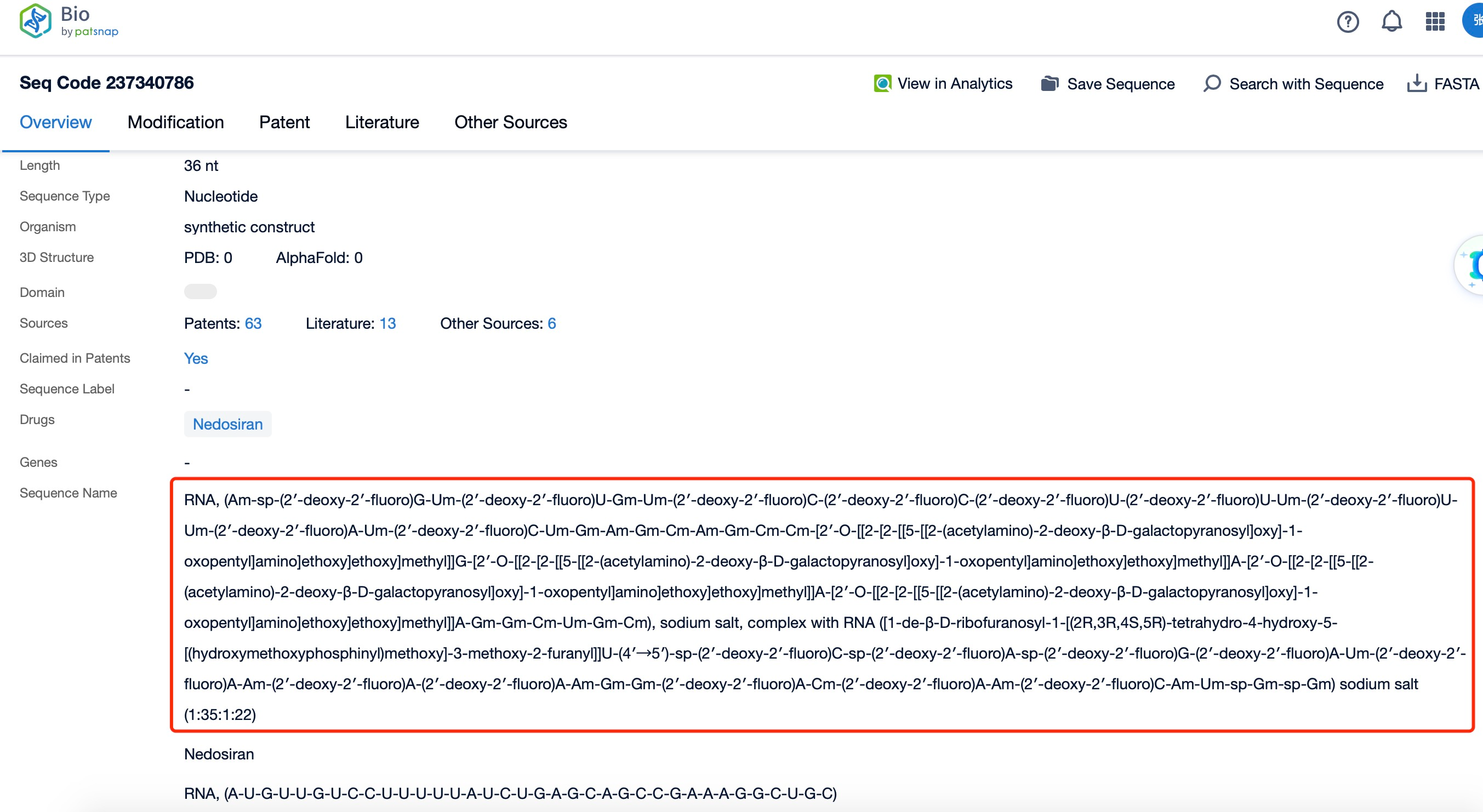
The chemical modification and action of nedosiran represent a sophisticated approach to RNAi therapeutics. The basic structure of the siRNA is enhanced through various chemical modifications to improve its drug-like properties. Common modifications include 2'-O-methyl (2'-OMe) and 2'-fluoro (2'-F) substitutions on the sugar rings, which increase nuclease resistance and reduce immunogenicity. Additionally, phosphorothioate linkages may be incorporated into the backbone to further enhance stability and facilitate cellular uptake.
The GalNAc conjugation is a key feature of nedosiran's chemical structure. This triantennary GalNAc moiety is typically attached to the 3' end of the sense strand via a linker molecule. The GalNAc ligand interacts with the asialoglycoprotein receptor on hepatocytes, facilitating rapid and efficient uptake of the siRNA into liver cells, where it can exert its therapeutic effect on LDHA expression.
Once internalized, nedosiran engages with the endogenous RNAi machinery. The siRNA is incorporated into the RISC, where the sense strand is discarded, and the antisense strand guides the complex to the complementary LDHA mRNA. The Argonaute 2 protein within RISC then cleaves the target mRNA, leading to its degradation and preventing the synthesis of the LDHA enzyme. This process effectively reduces oxalate production in the liver, addressing the root cause of PH1.
The chemical modifications not only enhance the stability and delivery of nedosiran but also contribute to its prolonged duration of action. By resisting degradation, the siRNA can persist in hepatocytes for extended periods, potentially allowing for less frequent dosing schedules. This is particularly beneficial for patients, as it may reduce the treatment burden and improve compliance.
Summary and Prospect
In summary, nedosiran represents a significant advancement in the treatment of primary hyperoxaluria type 1. As the second FDA-approved RNAi therapeutic for this rare genetic disorder, it offers a novel approach by targeting the LDHA gene to reduce oxalate production. The drug's approval marks a milestone in the field of RNAi therapeutics and provides new hope for PH1 patients who previously had limited treatment options.
The successful development and approval of nedosiran underscore the potential of RNAi technology in addressing previously challenging genetic disorders. Its liver-specific delivery mechanism and chemical modifications showcase the progress made in overcoming the hurdles traditionally associated with RNA-based therapeutics, such as stability and targeted delivery.
Looking to the future, the prospects for nedosiran and similar RNAi therapeutics are promising. Ongoing research will likely focus on long-term safety and efficacy data, as well as potential applications in other types of primary hyperoxaluria or related disorders. The success of nedosiran may also accelerate the development of RNAi therapies for other rare genetic diseases, particularly those involving liver-expressed genes.
Furthermore, the platform technology used in nedosiran's development could pave the way for more efficient and targeted RNAi therapeutics. As our understanding of the intricacies of RNA interference grows, we can anticipate further refinements in siRNA design, chemical modifications, and delivery strategies.
In conclusion, nedosiran stands as a testament to the power of translational research in bringing cutting-edge scientific discoveries to clinical practice. Its approval not only offers new hope for PH1 patients but also reinforces the potential of RNAi as a therapeutic modality. As research continues, we can expect to see an expanding landscape of RNAi-based treatments, potentially revolutionizing the approach to genetic and metabolic disorders.
How to find the chemical modification of all siRNAs?
In Patsnap Bio, you can find the sequence and latest research and development advances of all siRNAs.
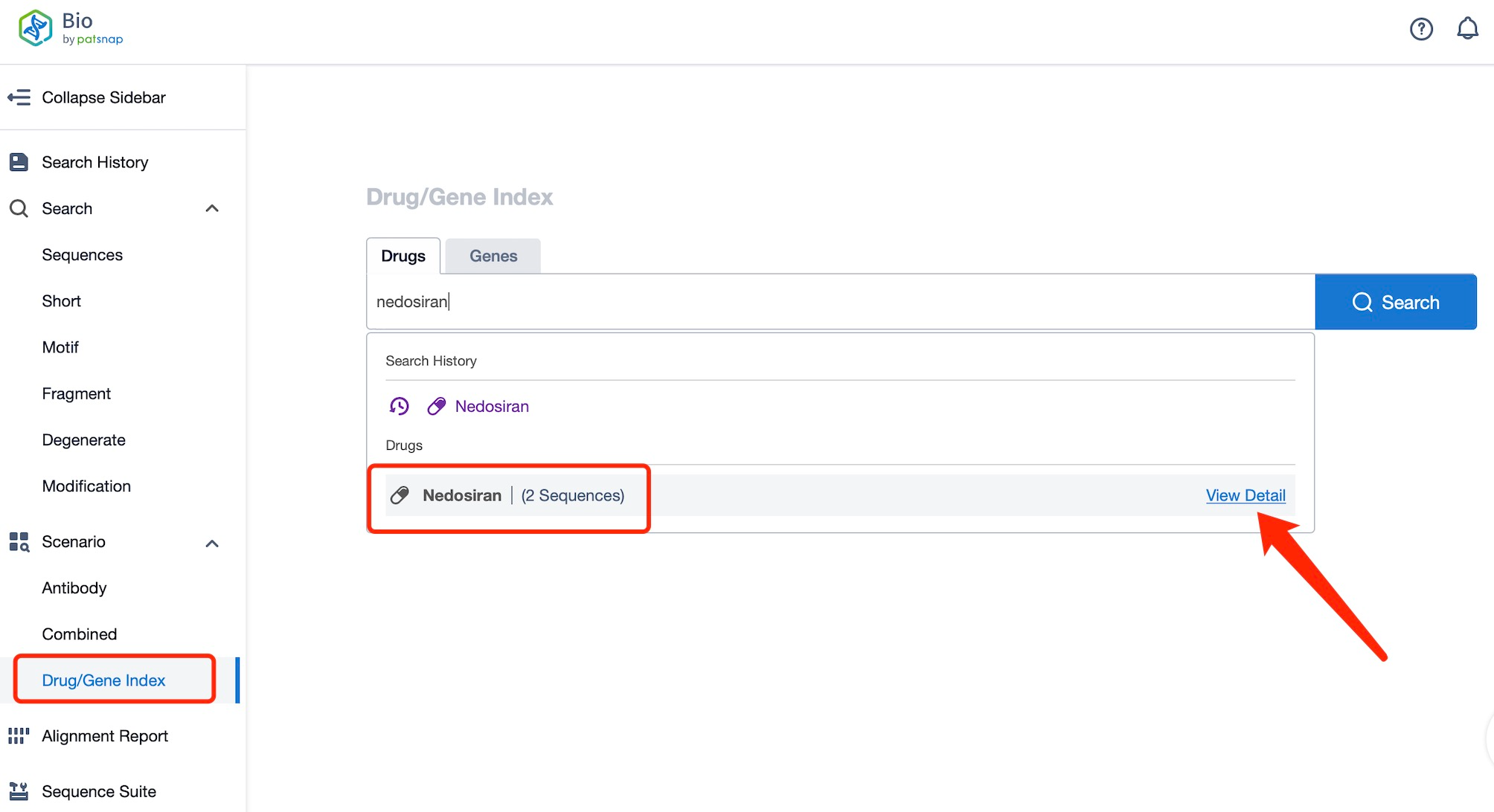
Taking nedosiran as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' nedosiran ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of nedosiran.
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
Clicking on the sequence name will provide you with all the basic information of that sequence.
Additionally, a visual diagram of the sequence's chemical modifications is available for immediate access.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.