How to gain an edge in BTK target research?
Currently, there are five marketed covalent irreversible inhibitors of Bruton's tyrosine kinase (BTK) (Figure 1), which achieve inhibition of BTK enzymatic activity by covalently binding to the C481 residue in the kinase domain of the BTK protein. Covalent irreversible BTK inhibitors have revolutionized the treatment of various B-cell malignancies, particularly chronic lymphocytic leukemia (CLL).
However, prolonged use of these covalent BTK inhibitors leads to the emergence of acquired resistance due to the development of C481 mutations in BTK (cysteine residue replaced by serine residue), resulting in diminished efficacy of these drugs. Non-covalent (reversible) BTK inhibitors overcome this mechanism of resistance and other resistance mechanisms, although the exact mechanisms of resistance to these therapies are not yet fully understood.
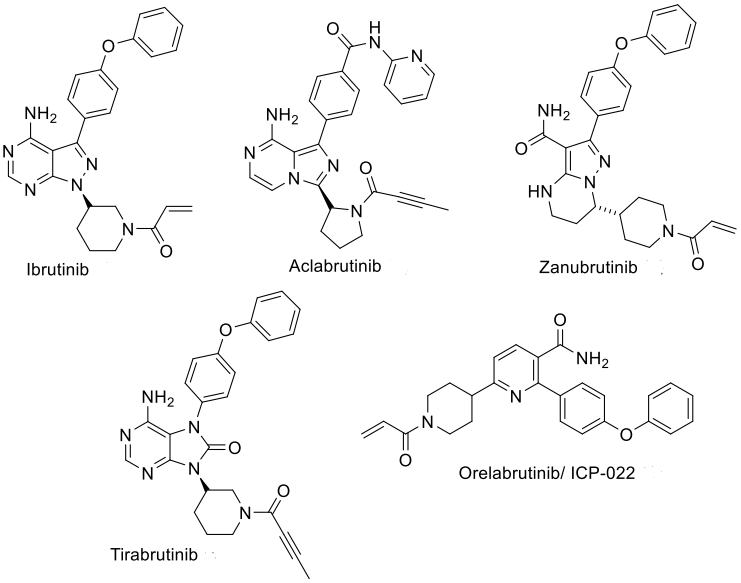
Since the approval of ibrutinib for the treatment of various hematologic tumors in 2013, efforts have been made to explore new BTK inhibitors. Although first-generation and second-generation irreversible BTK inhibitors have demonstrated significant potency and efficacy against various lymphomas and leukemias, there are also some clinical limitations, such as off-target toxicity and primary/acquired resistance.
Acquired resistance caused by the BTK C481S mutation is a major challenge for irreversible inhibitors. After the BTK C481S mutation, covalent irreversible inhibitors fail to form a covalent bond with BTK, resulting in reduced activity.
Therefore, there is an urgent need to develop new BTK inhibitors to overcome this mutation issue. In recent years, some reversible BTK inhibitors have been developed and are in various stages of evaluation.
In addition, reversible BTK-PROTACs have also entered clinical development. Among these reversible BTK inhibitors, pirtobrutinib from Loxo Oncology stands out as it was approved on January 27, 2023, for the treatment of relapsed or refractory mantle cell lymphoma (MCL) in adult patients who have received at least two prior lines of systemic therapy, including a BTK inhibitor.
The realm of BTK presents both challenges and opportunities. Why do major pharmaceutical companies love BTK? This is closely related to its immense market potential, wide range of indications, clinically prominent efficacy, and safety.
Why do major companies love BTK?
Firstly, there is a vast market potential. From the annual sales of ibrutinib at only $116 million in 2015, to the combined annual sales of ibrutinib, acalabrutinib, and zanubrutinib reaching $11.233 billion in 2022, the future market demand will further increase as clinical indications expand.
Secondly, BTK inhibitors have a wide range of indications. Taking the classic drug ibrutinib as an example, indications include: 1. Mantle cell lymphoma (MCL); 2. Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL); 3. CLL/SLL with 17p deletion; 4. Adult Waldenström's macroglobulinemia (WM); 5. Marginal zone lymphoma (MZL); 6. Chronic graft-versus-host disease (cGVHD). The indications approved in the United States include all of the above, while in China, it includes MCL/CLL/SLL. Some pharmaceutical companies are also attempting to rebuild the BTK inhibitor landscape in the field of autoimmune diseases.
Finally, BTK inhibitors exhibit excellent clinical efficacy and safety. For MCL, ibrutinib has an overall response rate (ORR) of 69%, and zanubrutinib has an ORR of 84.9%. For CLL/SLL, zanubrutinib has an ORR of 80.4%, and ibrutinib has an ORR of 72.9%.
In the final results of a phase III clinical trial that directly compared zanubrutinib and ibrutinib, zanubrutinib showed superior progression-free survival (PFS) and met the superiority hypothesis. For adult Waldenström's macroglobulinemia, in a phase III clinical trial comparing zanubrutinib to ibrutinib head-to-head, the experimental group achieved a combined complete response, very good partial response, and partial response rate (CR+VGPR+PR) of 77.5% compared to 77.8% in the control group, showing similar efficacy but better safety.
For marginal zone lymphoma, ibrutinib has a complete response and partial response rate (CR+PR) of 46%, while zanubrutinib has an ORR of 80%. Furthermore, the combination of BTK inhibitors with other approved drugs or therapies such as CAR-T is also being evaluated in clinical trials, which greatly expands the value of BTK inhibitors.
Does BTK have a future as a self-immune indication?
On June 30, 2022, the FDA suspended the Phase 3 clinical trials of Sanofi's tolebrutinib in multiple sclerosis and myasthenia gravis due to several cases of drug-induced liver injury. On December 23 of the same year, Novartis announced that the FDA has implemented a partial clinical hold on orelabrutinib for the treatment of multiple sclerosis, and no new patient recruitment will take place in ongoing Phase 2 studies in the United States.
The issue of liver injury caused by BTK inhibitors has cast a shadow over the exploration of BTK inhibitors in the field of self-immune indications. When using cancer drugs to treat chronic diseases, safety is a primary concern because chronic diseases require long-term medication, and the safety requirements are much higher than those of cancer drugs.
Sanofi believes that tolebrutinib has limited impact on individuals with normal liver function and has modified the clinical trial protocol by excluding patients with pre-existing liver impairment and increasing the frequency of liver function monitoring in enrolled patients.
Further clinical data disclosure is needed to determine whether the occurrence of liver injury is due to factors related to multiple sclerosis patients themselves or issues with the drug molecule. If it is a problem with the molecule itself, further optimization such as improving efficacy and reducing dosage may be required to minimize side effects.
However, there is also another possibility of ineffectiveness at the mechanistic level. The reason BTK inhibitors can treat tumors is because they can inhibit the generation of new B cells, thereby preventing disease progression.
However, this mechanism may not be effective for autoimmune diseases because BTK inhibitors lack the ability to target existing B cells and immune cells that are responsible for causing immune diseases. In fact, several BTK inhibitors from various multinational companies have encountered setbacks in the field of autoimmune diseases.
Whether this "ineffectiveness theory" is valid or not remains to be revealed through fundamental research. Risks and opportunities coexist, and currently, there is not enough evidence to indicate that mechanistic deficiencies are the cause of BTK inhibitors' inability to be used as self-immune indications.
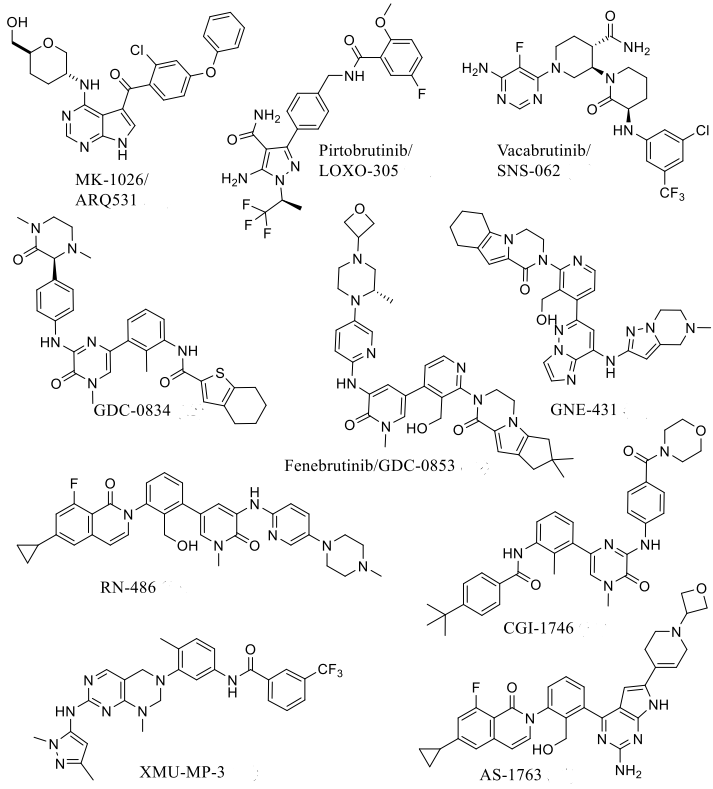
One of the directions for breakthrough: Reversible non-covalent BTK inhibitors targeting the C481S mutation
Non-covalent inhibitors of BTK are superior to covalent inhibitors. Due to the resistance caused by the Cys481 mutation, covalent inhibitors lose their activity, while non-covalent inhibitors retain their potency against the C481S and C481R BTK variants.
Reversible non-covalent inhibitors remain an effective treatment option for patients resistant to ibrutinib due to mutations in Cys481 or Thr474. The chemical structure of reversible non-covalent BTK inhibitors specifically targeting the C481S mutation is shown in Figure 2, with a summary of in vitro enzyme inhibition and cellular inhibition data for reversible BTK inhibitors provided in Table 1.
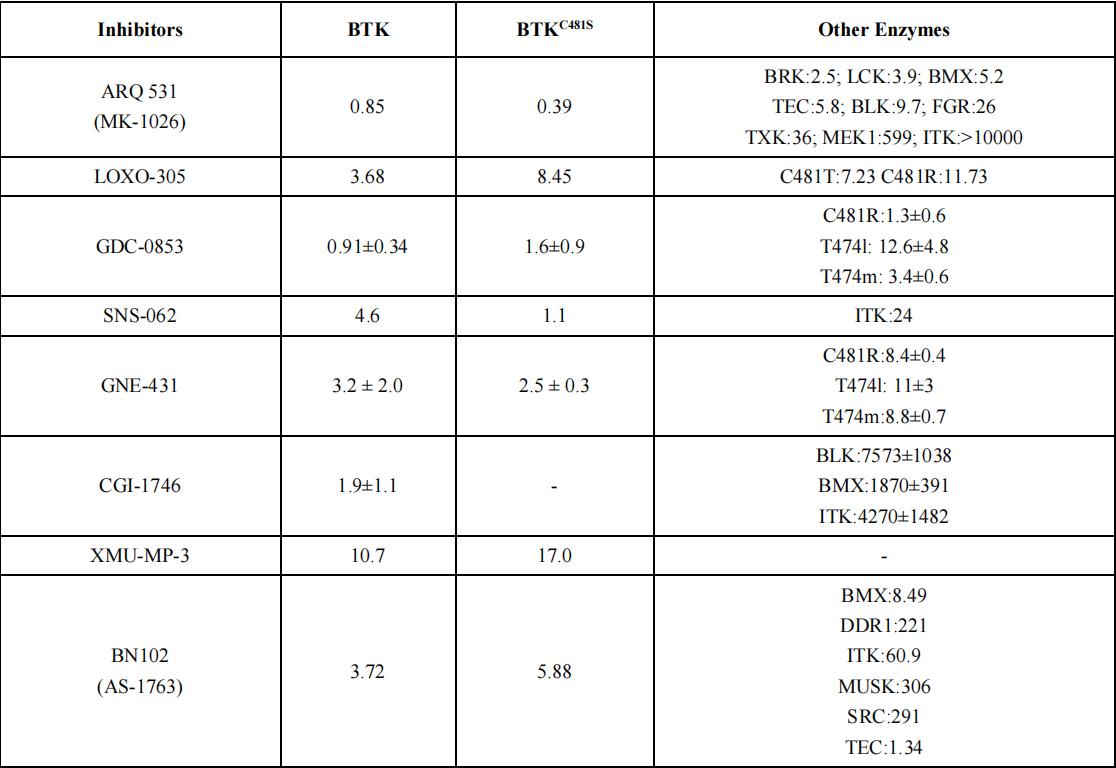
Reversible, non-covalent next-generation BTK inhibitors have advantages over existing covalent inhibitors and exhibit lower toxicity compared to irreversible TKIs. Eli Lilly's pirtobrutinib (LOXO305) emerged as a frontrunner and was approved for market on January 27, 2023.
However, subsequent non-covalent molecules face significant challenges in surpassing LOXO305. Differentiation can only be sought through avenues such as efficacy, safety, and exploration of new indications.
Summary
BTK has been a prominent target in medicinal chemistry research, and we have witnessed the development of next-generation BTK inhibitors in recent years. While ibrutinib has shown good clinical efficacy, off-target toxicity and acquired/primary resistance associated with its common shortcomings have led to the clinical development of second-generation BTK inhibitors, such as acalabrutinib and zanubrutinib, which possess higher selectivity and reduced off-target toxicity.
BTK C481 mutation is the primary cause of acquired resistance to first-generation and second-generation covalent BTK inhibitors. In recent years, many BTK inhibitors, including irreversible, reversible, reversible covalent, and BTK PROTACs, have been identified and developed. Next-generation BTK inhibitors primarily aim to overcome the BTK C481S mutation.
Additionally, BTK inhibitors are being investigated in clinical studies for the treatment of autoimmune diseases. Although BTK inhibitors face challenges in the field of autoimmunity, further clinical data is needed to determine whether the issues lie with the drug molecules themselves or the mechanisms involved.

References
1.Jian Hong,et al, Next-generation Bruton’s Tyrosine Kinase (BTK) Inhibitors Potentially Targeting BTK C481S Mutation- Recent Developments and Perspectives.DOI: 10.2174/1568026622666220801101706.
2.Eric Wang,et al, Mechanisms of Resistance to Noncovalent Bruton’s Tyrosine Kinase Inhibitors.N Engl J Med. 2022 February 24; 386(8): 735–743.