How to search for global clinical trial information on drugs?
Information on ongoing clinical drug trials worldwide can be accessed through the following channels:
ClinicalTrials.gov: This is a database maintained by the U.S. National Institutes of Health (NIH) and provides information about clinical trials registered from around the world. You can find various types and phases of clinical trials with a simple keyword search, such as medication, disease name, study location, etc., and view detailed information about each trial, including design, eligibility criteria, progress, and results (if published).
WHO International Clinical Trials Registry Platform (ICTRP): The ICTRP of the World Health Organization (WHO) is a global clinical trial registration platform that aggregates data from major registries around the world, offering a path to search for global clinical trials.
European Clinical Trials Database (EudraCT): For clinical trials conducted in the member states of the European Union, this database provides pathways for searching and accessing. It includes all the information on clinical trials of medicinal products, from phase I to later-stage trials.
China Clinical Trial Registration Center (ChiCTR): This is a registration and disclosure platform in China, dedicated to the public and transparent sharing of information on clinical trials conducted in China.
When using the above databases, you can accurately find the information you need using a range of filters such as drug name, indications, trial phase, trial status, sponsor, type of study, and geographic location. These databases usually allow free access and provide search interfaces in English, as well as in the local language where possible. It should be noted that although these databases strive to contain comprehensive and up-to-date clinical trial information, there may be overlaps among them, and not all ongoing clinical trials are registered in these databases. Therefore, if one needs as comprehensive information as possible, it may be necessary to search multiple registries.
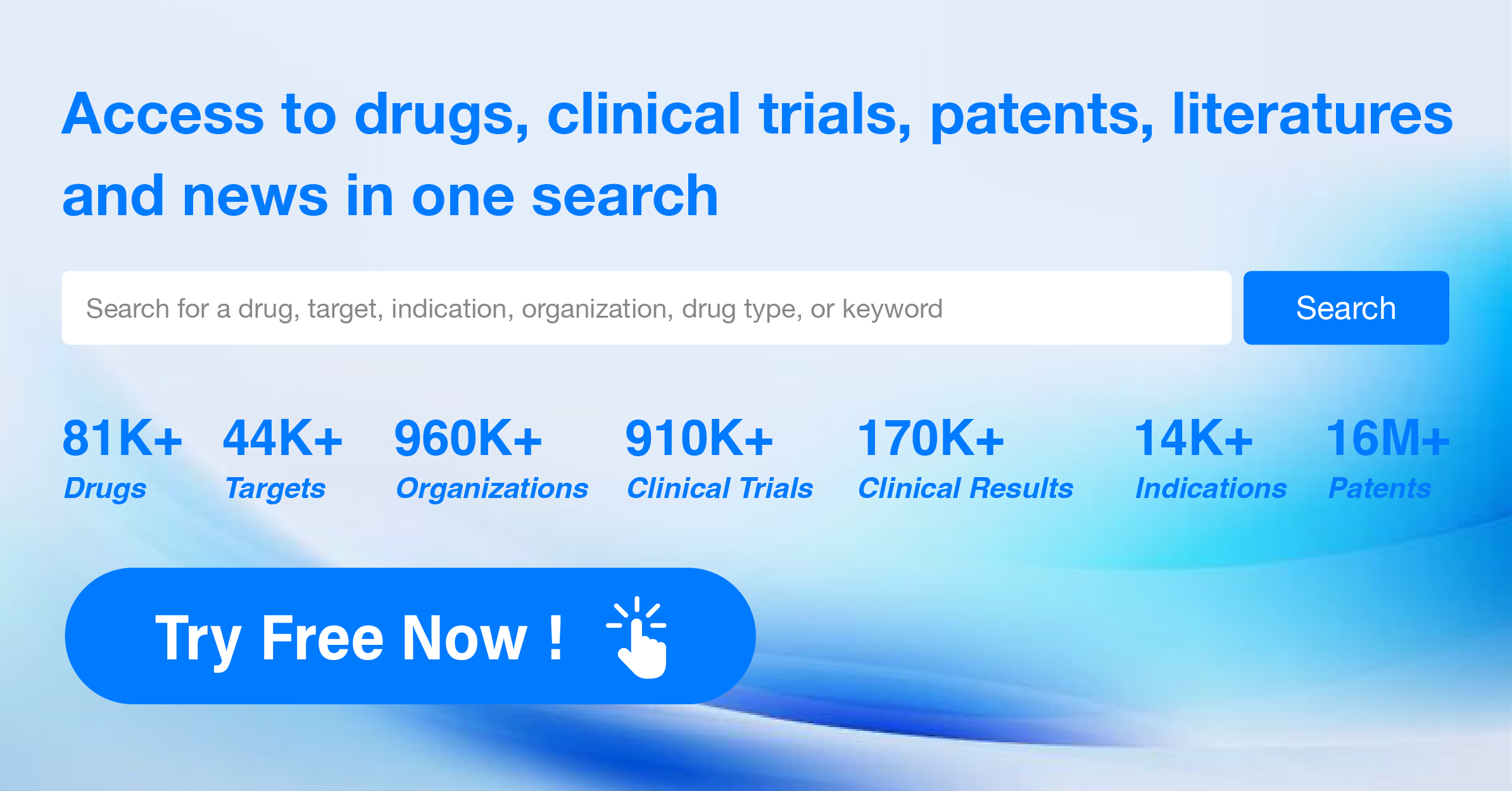
Here, we recommend Synapse, a professional global pharmaceutical data website. Synapse, as a data product focused on innovative drugs, pays close attention to the global pharmaceutical industry, highly integrating and standardizing global drug patent information, registration filings, academic literature, clinical information, news intelligence, and market sales. It has built a unique mapping system and tracks drug concerns through multiple channels such as patents, transactions, financing, and company announcements, providing the fastest and latest data on global new drugs.
For the problem of difficulty in following up on pipeline/trade/clinical results information, one can subscribe to the content of interest within the Synapse database to receive update alerts in personalized intelligence subscriptions, thus staying up to date with the latest R&D developments.