Initial Results from Phase 2 Trial of STAT3 Inhibitor and Radiation in Glioblastoma Treatment
Moleculin Biotech, Inc., a Phase 3 clinical-stage pharmaceutical firm with a wide array of drug candidates aimed at difficult-to-treat tumors and viruses, has declared the enrollment and commencement of patient treatment in an Investigator-initiated Phase 2 study. This study is evaluating the efficacy of WP1066 combined with radiation therapy for treating adult glioblastoma (NU 21C06).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The NU 21C06 trial is a Phase 2, open-label, multi-arm investigation examining radiation therapy paired with WP1066 for newly diagnosed IDH wild-type, MGMT-unmethylated glioblastoma patients. The primary endpoint of the study is progression-free survival, while secondary endpoints include analysis of the tumor microenvironment.
Dr. Priya Kumthekar, an Associate Professor at Northwestern University and Co-Investigator for the trial, remarked, “Initiating patient dosing is a crucial milestone toward addressing the significant unmet need in glioblastoma treatment. The preclinical data were highly promising, showing that WP1066 combined with radiation therapy extended survival and triggered anti-tumor immune responses. We are eager to advance to patient treatment with this novel strategy.”
Dr. Amy Heimberger, Co-Investigator and Vice Chair for Research at the Department of Neurological Surgery, Northwestern University, stated, “The urgency of this unmet need is reflected in the rapid recruitment for this trial. Within the initial months of the trial’s commencement, four participants are already involved, and we anticipate soon filling the safety lead-in cohort of six subjects. Should the treatment prove tolerable for these participants, we will proceed with further enrollment.”
WP1066 is the leading Immune/Transcription Modulator from Moleculin, engineered to enhance immune responses against tumors by blocking the aberrant activity of regulatory T cells and inhibiting key oncogenic transcription factors such as p-STAT3, c-Myc, and HIF-1α. These transcription factors are crucial targets due to their roles in cancer cell survival, growth, angiogenesis, invasion, metastasis, and tumor-related inflammation.
Mr. Walter Klemp, Chairman and CEO of Moleculin, commented, “The NU 21C06 study is an essential step in advancing technologies that target STAT3. STAT3 has been a highly sought-after but elusive target in cancer therapy. Our collaborative efforts with Northwestern University, supported by the NIH and BrainUp®, could potentially revolutionize this area. Based on current data, we believe WP1066 has the potential for significant anti-tumor effects across various hard-to-treat cancers.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
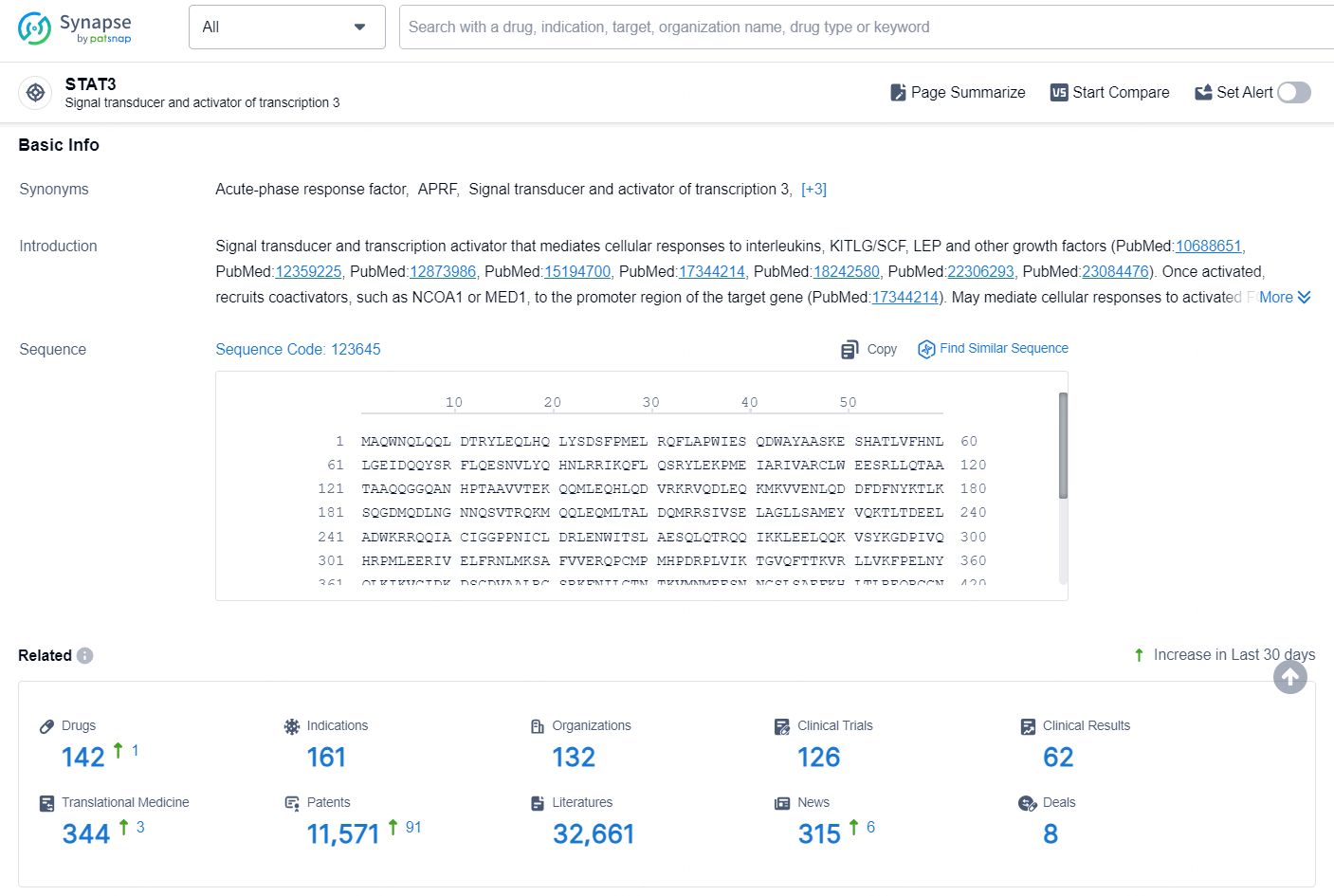 According to the data provided by the Synapse Database, As of September 14, 2024, there are 142 investigational drugs for the STAT3 target, including 161 indications, 132 R&D institutions involved, with related clinical trials reaching 126, and as many as 11571 patents.
According to the data provided by the Synapse Database, As of September 14, 2024, there are 142 investigational drugs for the STAT3 target, including 161 indications, 132 R&D institutions involved, with related clinical trials reaching 126, and as many as 11571 patents.
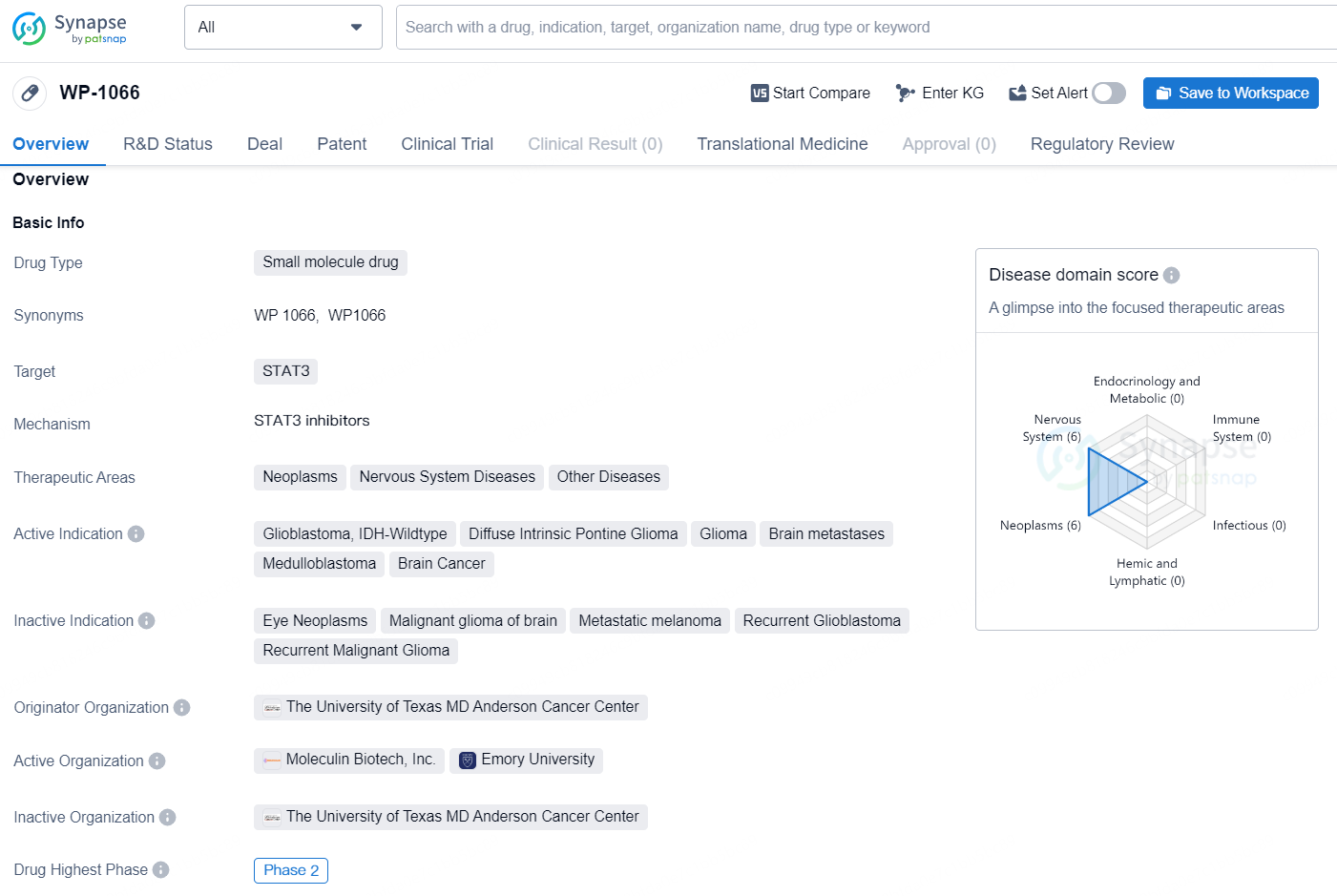
The drug WP-1066 is a small molecule drug that targets STAT3 and is being developed for the treatment of various therapeutic areas including neoplasms, nervous system diseases, and other diseases. It is actively indicated for the treatment of glioblastoma, IDH-Wildtype, diffuse intrinsic pontine glioma, glioma, brain metastases, medulloblastoma, and brain cancer.