Japan's Department of Health sanctions CSL and Arcturus's ARCT-154, an initial mRNA booster for adult COVID-19 authorization
Internationally recognized biotech company CSL, in partnership with Arcturus Therapeutics, has made public that the Ministry of Health, Labor and Welfare in Japan has officially sanctioned the use of ARCT-154. This novel self-amplifying mRNA vaccine is designed to combat COVID-19 and is authorized for primary immunization as well as reinforcement doses in individuals aged 18 and above.
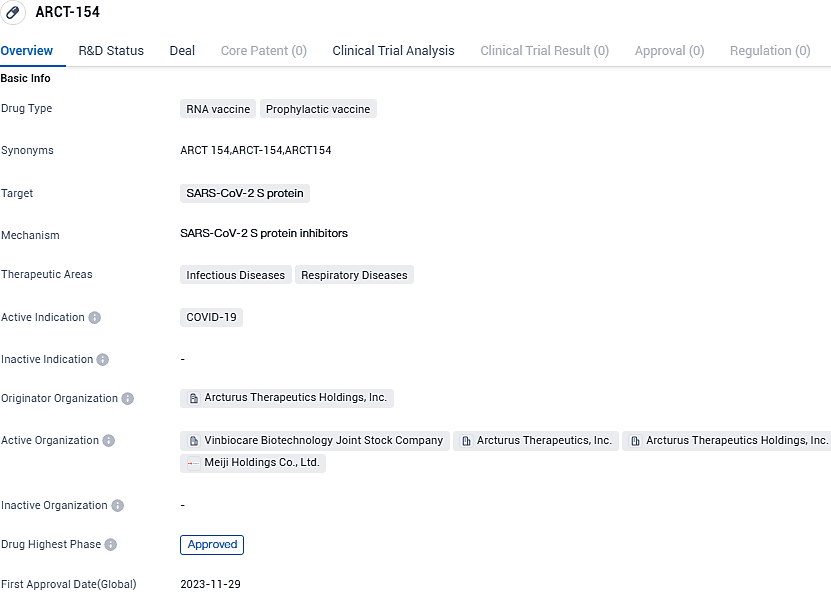
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The innovative self-amplifying mRNA technology holds promise as a long-term immunization strategy, according to Nobel Prize recipient Dr. Drew Weissman. "I am eager to witness how this advanced form of mRNA technology will offer a shield against COVID-19 and potentially a range of other serious infectious agents."
Dr. Jonathan Edelman, CSA's Senior Vice President of the Vaccines Innovation Unit expressed his excitement about the groundbreaking authorization of the world's premier sa-mRNA vaccine. "CSL is dedicated to fulfilling its commitment to safeguarding worldwide health, and this development is a testament to that. We are working closely with health agencies around the globe to make this crucial vaccine technology accessible for those vulnerable to COVID-19," he stated.
The green light for the vaccine follows robust clinical trial results from various ARCT-154 research, which encompasses a significant efficacy trial ongoing in Vietnam with 16,000 participants, along with a Phase 3 COVID-19 booster study. The latter demonstrated stronger immunogenicity and a satisfactory safety profile when compared to an established mRNA COVID-19 vaccine benchmark. Preliminary data have been disseminated via MedRxiv and are slated for inclusion in a reputable scientific journal before the year concludes.
Arcturus Therapeutics' CEO, Joseph Payne, shared his pride in the company's contribution to the creation and validation of the inaugural sa-mRNA product to gain approval. "The greenlighting of our sa-mRNA vaccine against COVID-19 marks a crucial milestone. Anticipating future projects, we are thrilled to pursue novel applications of our proprietary sa-mRNA vaccine technology in partnership with our exclusive global ally, CSL."
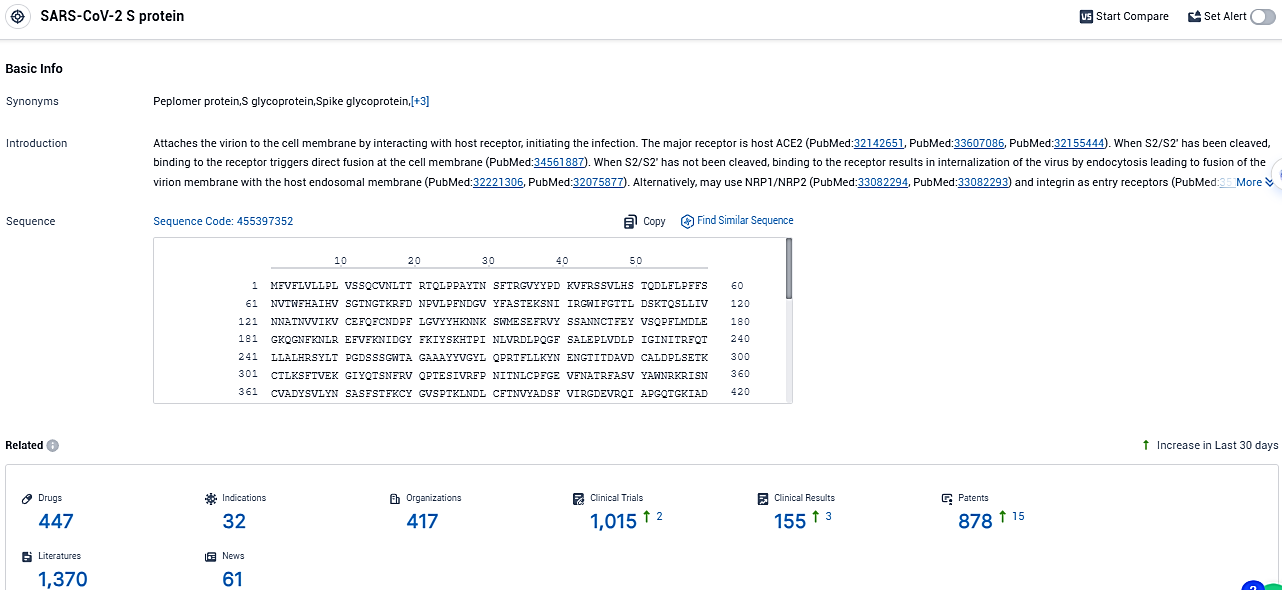
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 6, 2023, there are 447 investigational drugs for the SARS-CoV-2 S protein target, including 32 indications, 417 R&D institutions involved, with related clinical trials reaching 1015, and as many as 878 patents.
The development of ARCT-154 represents a significant advancement in the field of biomedicine, particularly in the context of the ongoing COVID-19 pandemic. By specifically targeting the SARS-CoV-2 S protein, the vaccine aims to provide protection against the virus and potentially reduce the severity of COVID-19 symptoms. The approval of ARCT-154 in Japan will mark a crucial milestone in the fight against COVID-19, offering a potential solution to combat the infectious and respiratory diseases caused by the virus.