KaliVir: A New Oncolytic Virus Therapy Targeting CXCR3/CCR2
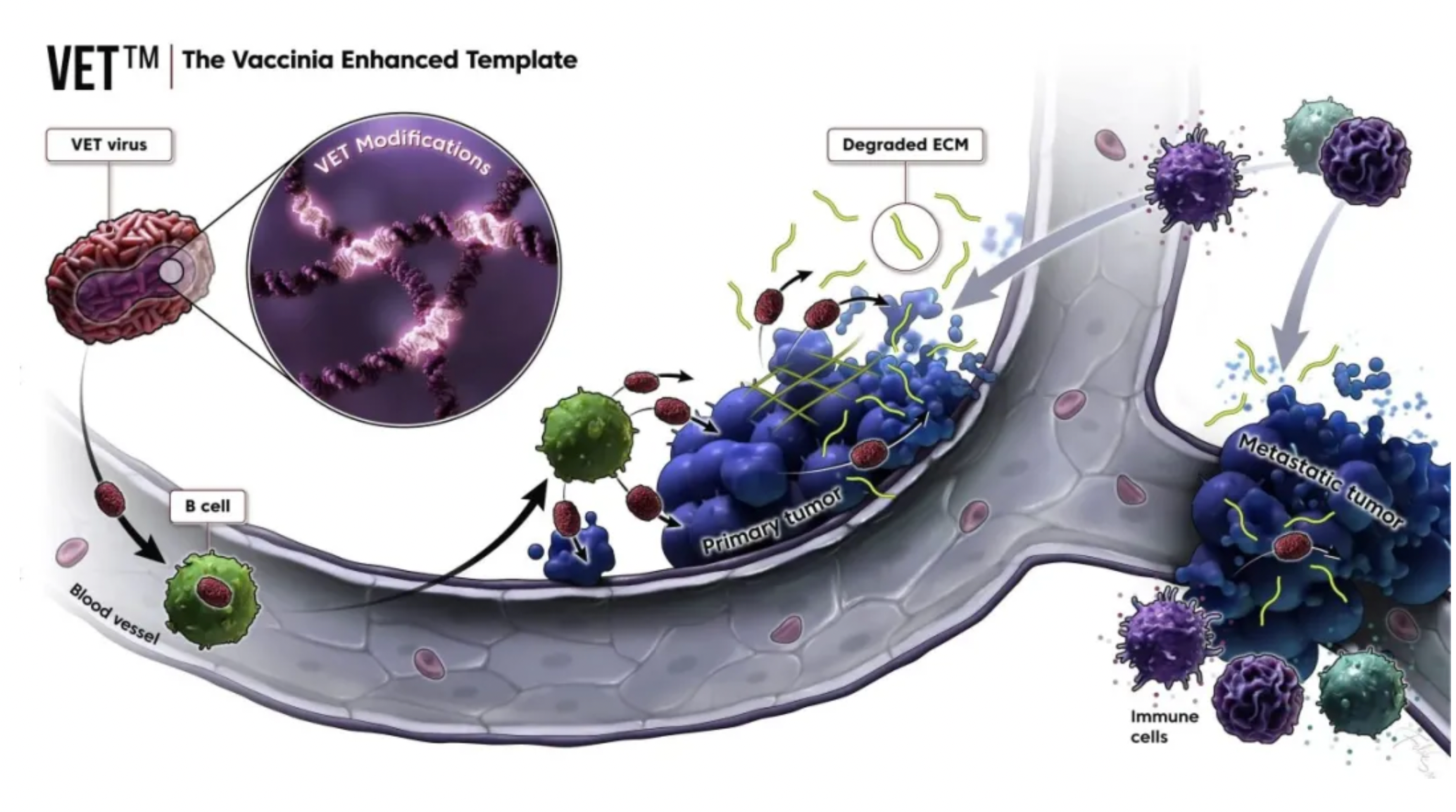
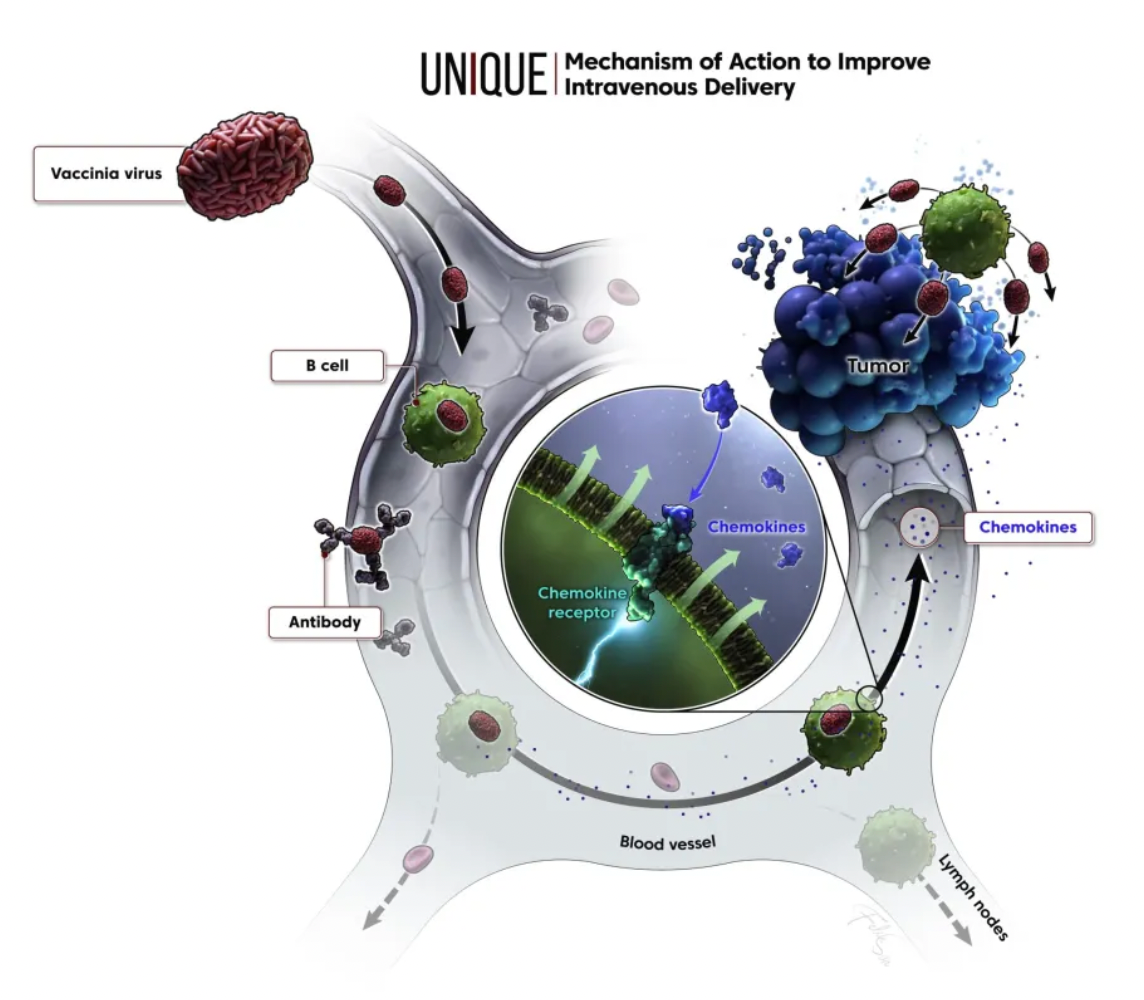
KaliVir Immunotherapeutics (KaliVir) is a biotechnology company focused on the development of cutting-edge, multi-mechanism oncolytic virus immunotherapy programs. Currently, based on the company’s Vaccinia virus platform known as the Vaccinia Enhanced Template (VET™) Platform, KaliVir is able to produce novel, potent oncolytic Vaccinia viruses with high replication ability and enhanced capabilities for intravenous administration and dissemination.
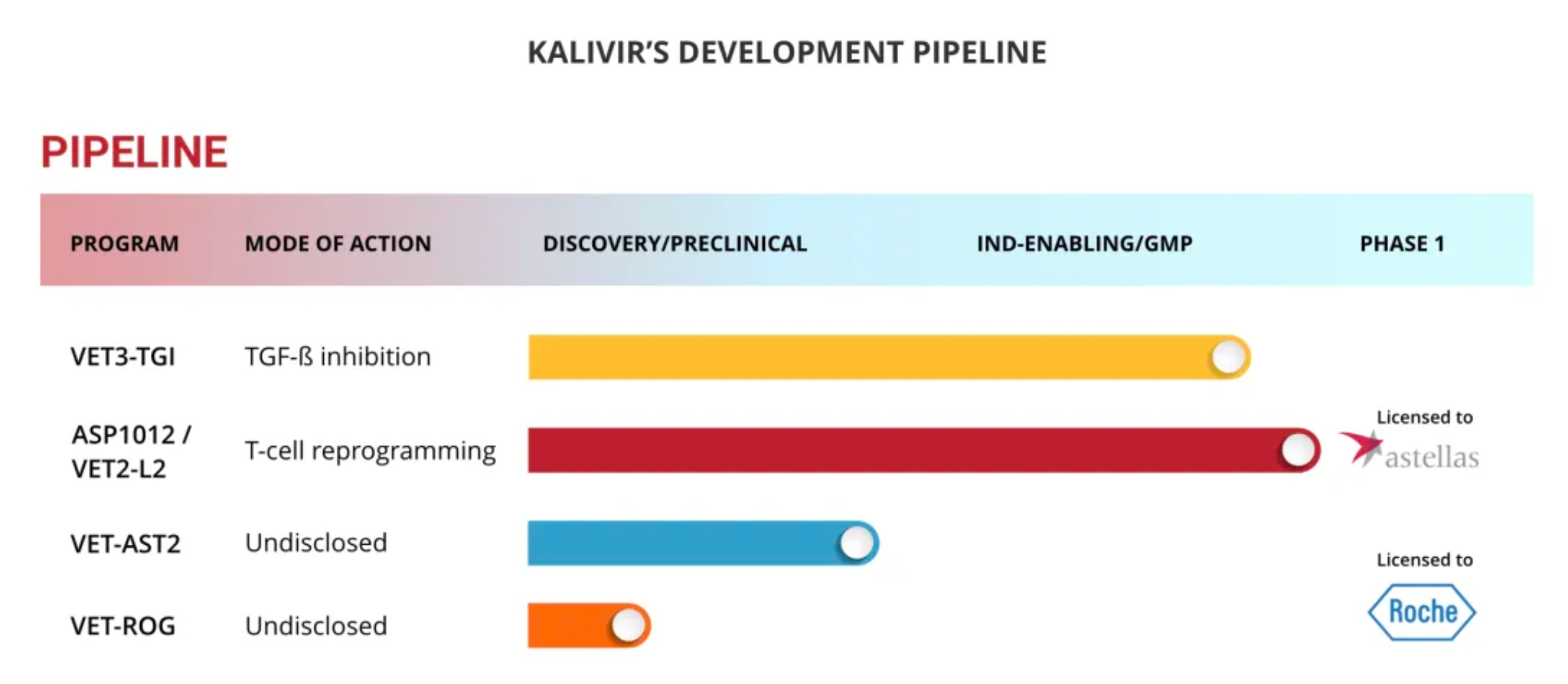
The VET™ platform incorporates multiple proprietary genetic modifications, which can be combined to create a unique oncolytic virus. This virus is optimized for systemic administration and expresses therapeutic transgenes within tumors. The mechanisms of the VET™ platform may include immunomodulation aimed specifically at enhancing anti-tumor immunity.
The design objectives of the VET™ platform include:
·Enhancing systemic (intravenous) administration capabilities;
·Enhancing the replication ability targeted at tumors;
·Enhancing the virus’s spread to metastatic tumors;
·Enhancing viral dissemination within the tumor microenvironment.
Using VET3-TGI as an example, this candidate product is a novel oncolytic immunotherapy that, in preclinical studies, specifically targets and preferentially kills tumor cells, while stimulating anti-tumor immunity through the expression of transgenes containing interleukin-12 (IL-12) and TGFβ inhibitors. Simultaneously, the virus expresses CXCR3 to enhance systemic viral delivery to tumors rich in CXCR3 ligands and locally expresses IL-12 and TGFβi in the tumor microenvironment, effectively controlling various tumor models.
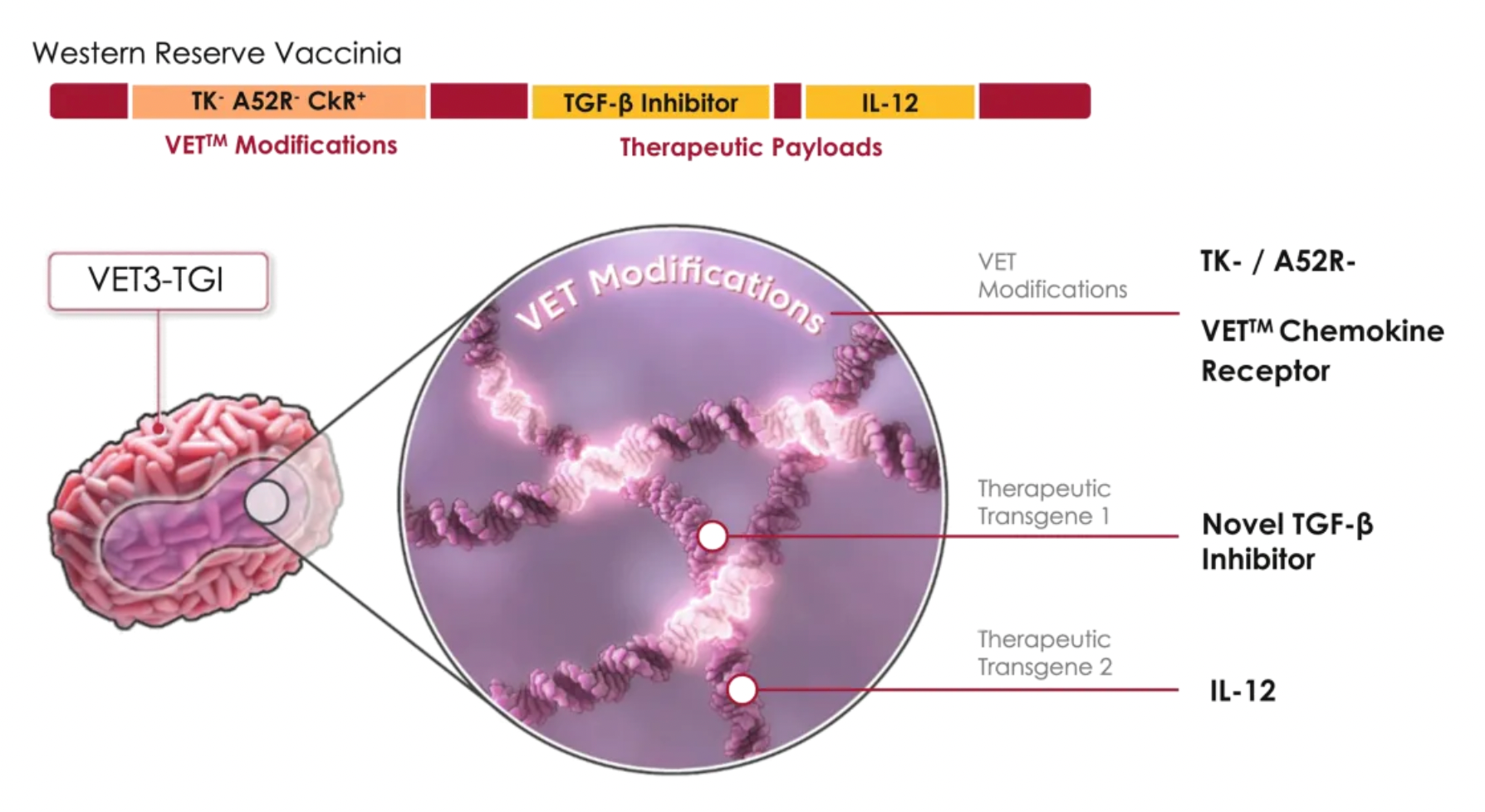
In early 2024, the company obtained approval from the U.S. FDA for an Investigational New Drug application for the STEALTH-001 study using VET3-TGI for the treatment of incurable advanced solid tumors, and the project is currently in Phase 1 clinical trials. This project (ClinicalTrials.gov identifier NCT06444815) will evaluate the safety and efficacy of VET3-TGI administered via intravenous infusion or intratumoral injection, assessing the therapeutic effects of VET3-TGI as a monotherapy and in combination with immune checkpoint inhibitors.
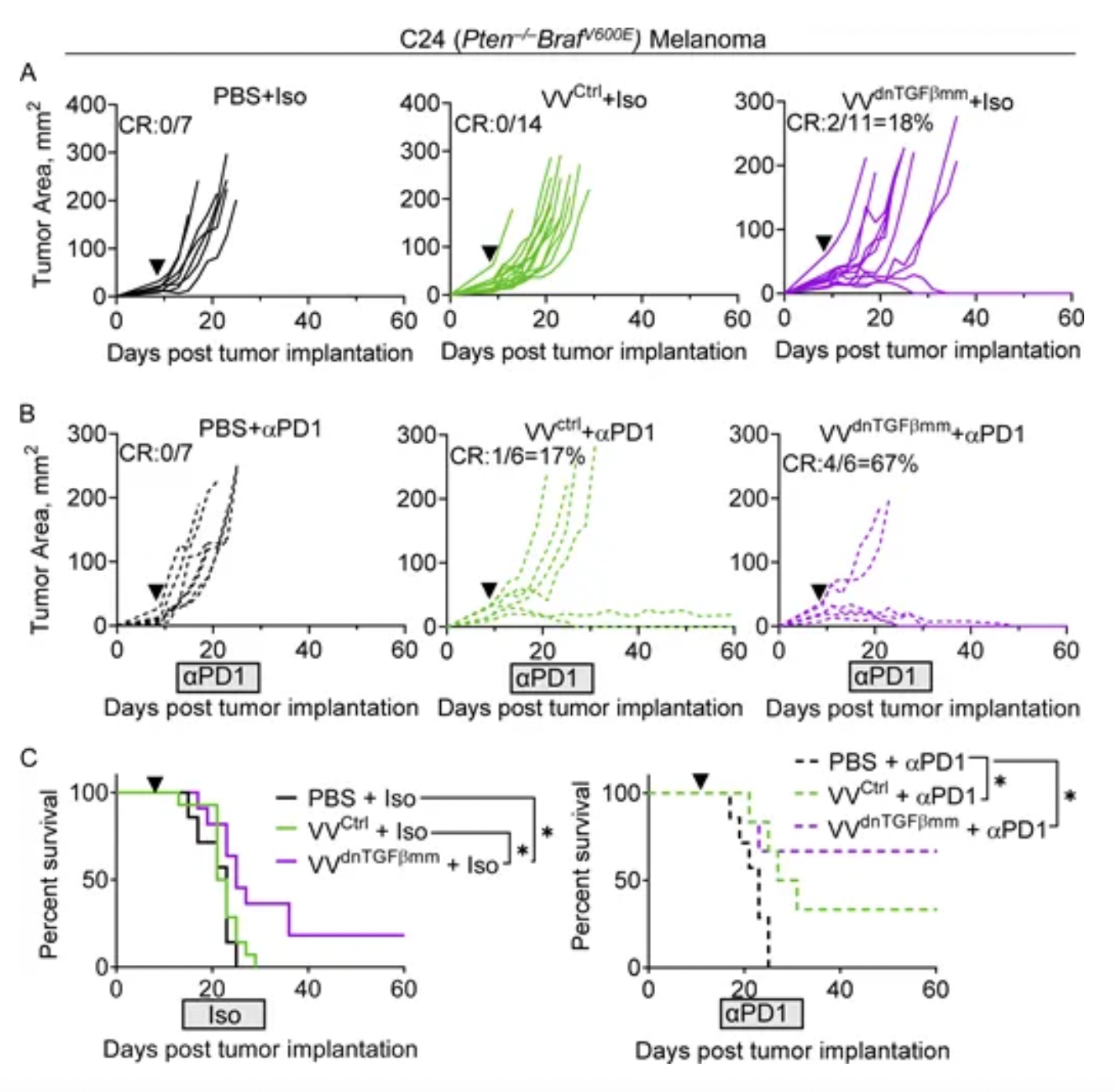
According to a research poster abstract, cells infected with VET3-TGI express CXCR3 and demonstrate enhanced migratory capacity towards CXCR3 ligands in vitro, retaining enhanced systemic delivery to CXCR3 ligand-expressing tumors in vivo despite pre-existing antiviral immunity. In vitro evidence confirmed IL-12 expression and TGFβi blocked TGFB1's inhibitory effect on CD8 T-cell proliferation. In vivo studies in mice using EMT6, RENCA, and MC38 tumor models showed robust therapeutic activity, achieving 100% complete remission rates even at doses far below equivalent clinical doses, with this effect observed across multiple models.
In another preclinical research model published by the KaliVir team, an oncolytic virus delivering TGFβ inhibitors by VET™ can overcome the immunosuppressive tumor microenvironment, achieving synergistic effects with anti-PD1 treatment.
DOI: 10.1084/jem.20230053
In addition to VET3-TGI, the company has several other programs in clinical or pre-clinical development stages. These include ASP1012, which is licensed exclusively to Anstelai Pharmaceuticals and involves delivering oncolytic viruses through the expression of the CCR2 receptor; and VET-ROG, developed in partnership with Roche (details not disclosed).
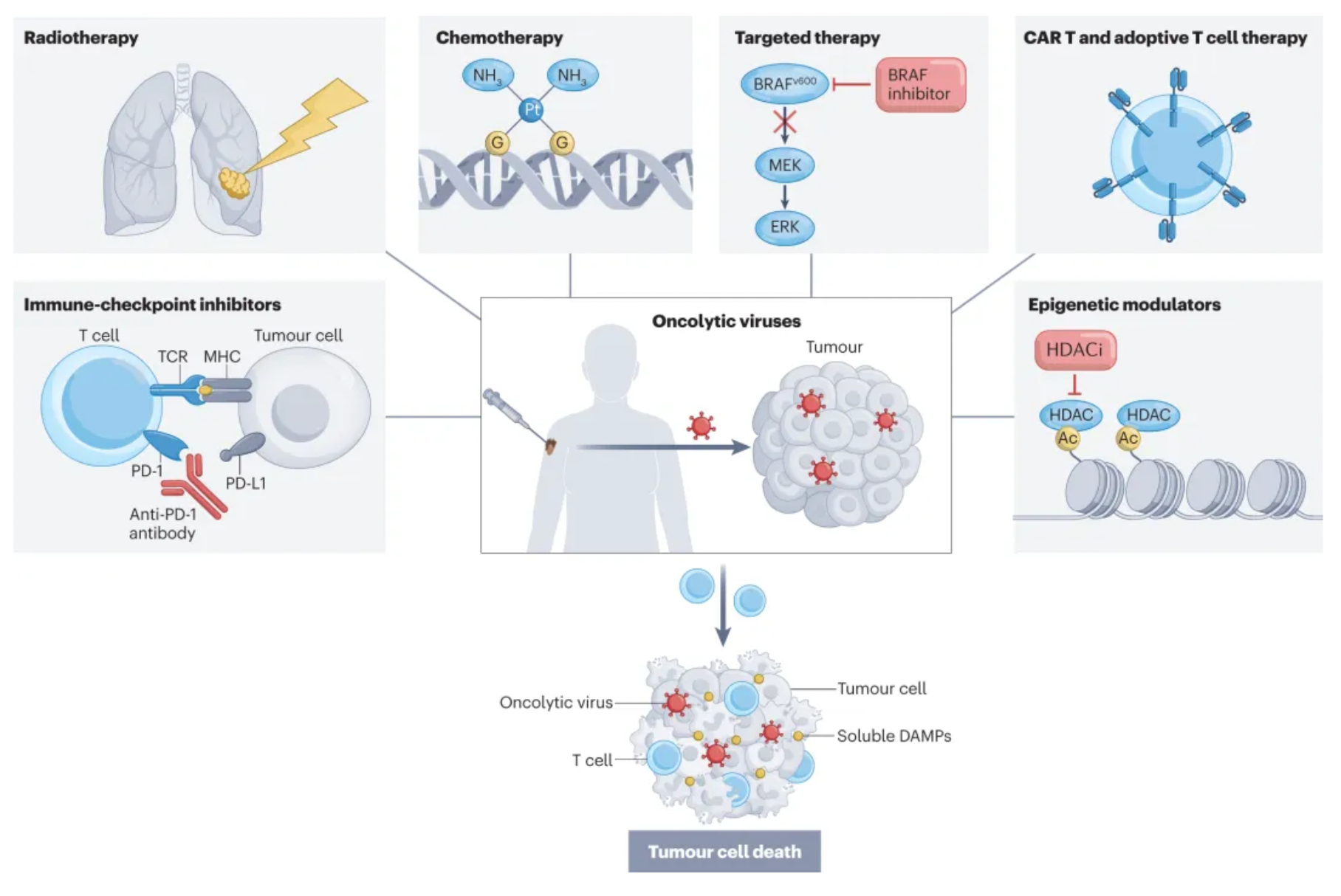
Oncolytic viruses (OVs) are an emerging class of cancer therapy that offer benefits including selective replication in tumor cells, delivery of various eukaryotic transgene payloads, induction of immunogenic cell death, and enhancement of anti-tumor immunity. They also possess a tolerable safety profile that can complement other cancer treatment modalities extensively.
Talimogene laherparepvec (T-VEC) is the world's first engineered oncolytic herpes simplex virus type 1 (HSV1), designed to preferentially replicate in tumor cells and induce an anti-tumor immune response. A prospective randomized trial evaluated the intratumoral injection of T-VEC, achieving its primary endpoints by enhancing the durable response rate in patients with palpable and unresectable melanoma, and showed improvements in objective response rate (ORR), progression-free survival (PFS), and overall survival (OS). Consequently, in 2015, it received full approval from the FDA.
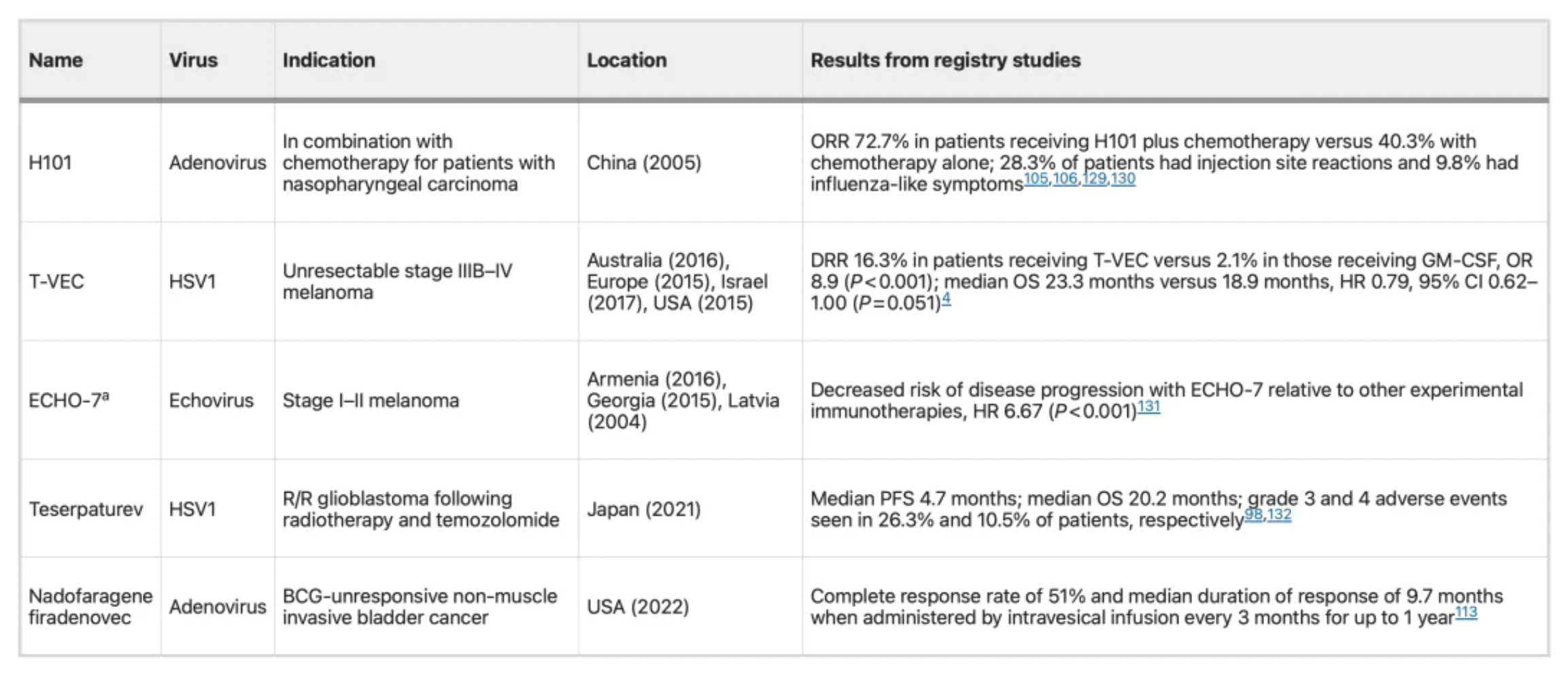
Below is a table of various oncolytic virus products that have been approved for use globally.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!