Lirum BioMed's LX-101 Shows Promise Against IGF-Linked Tumors,Set for 2024 ESMO Spotlight
Lirum Therapeutics, Inc., a pioneering enterprise operating in the clinical-phase biopharmaceutical industry, is dedicated to developing treatments for severe illnesses. The company has recently reported that its compelling findings regarding LX-101, an innovative therapy in clinical trials that is designed to specifically target the IGF-1R, will be showcased at the upcoming 2024 ESMO Congress for Sarcoma and Rare Cancers. This significant event is scheduled to unfold in Lugano, Switzerland, between the dates of March 14th and 16th.
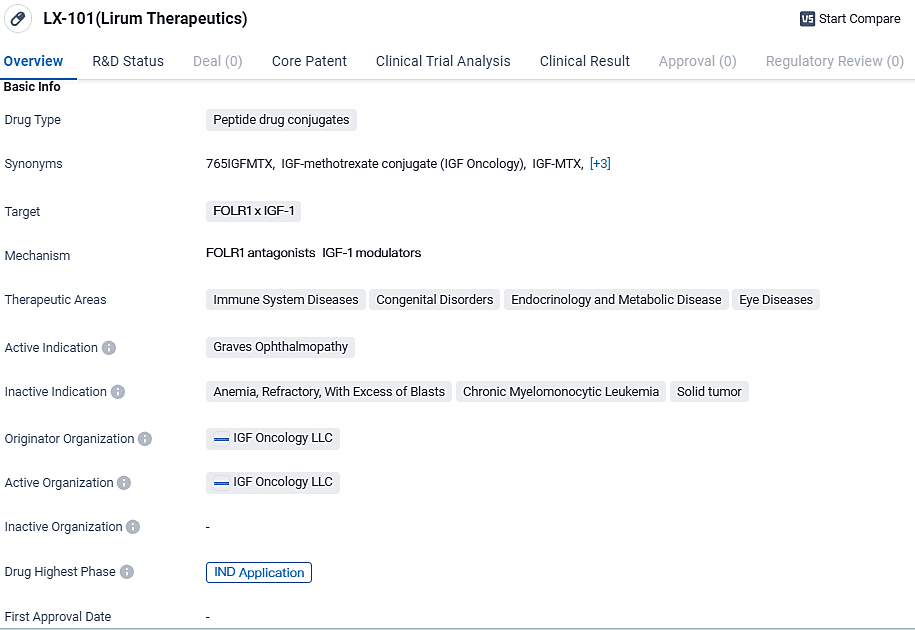
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Lirum's research encompasses clinically substantiated reports of LX-101 demonstrating significant preclinical efficacy in combating tumors that are known to involve the IGF-1R signaling pathway. This includes cancer types that exhibit genetic changes impacting the IGF-1R pathway directly or have an elevated expression of IGF-1R.
In light of these encouraging findings and the supporting clinical evidence, Lirum is currently strategizing future clinical studies to assess LX-101 in treating various cancer forms, in both children and adults, that are notably connected with aberrations in the IGF-1/IGF-1R axis. Additionally, Lirum is exploring the potential of LX-101 as a treatment for specific autoimmune conditions, such as thyroid dye disease, where the role of IGF-1R has been both clinically and commercially acknowledged.
Regarding peptide-based therapeutics, these compounds integrate peptides with other chemical entities to amplify their medicinal impact. In the case of LX-101, its molecular design specifically targets the convergence of FOLR1 and IGF-1. FOLR1 is a targeted receptor found in excess on certain tumor cells, making it an attractive molecular target for precision therapies. Conversely, IGF-1 is an influential factor in cellular development and multiplication.
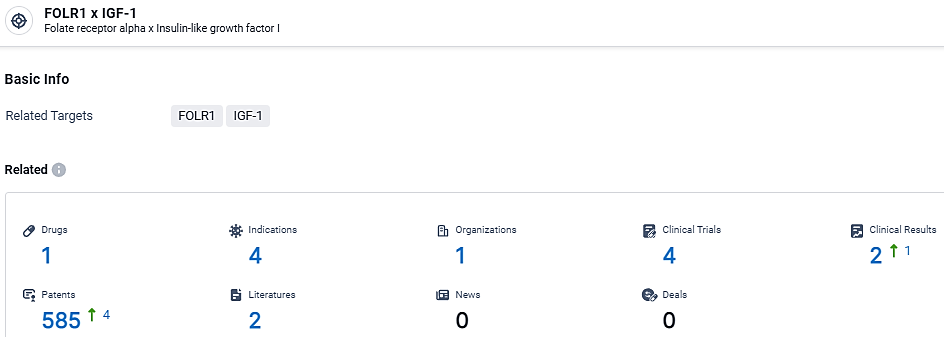
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 18, 2024, there are 1 investigational drugs for the FOLR1 and IGF-1 target, including 4 indications, 1 R&D institutions involved, with related clinical trials reaching 4, and as many as 585 patents.
LX-101 shows promise as a potential treatment for Graves Ophthalmopathy and potentially other diseases within its therapeutic areas. As it progresses through the IND application phase, further clinical trials will be conducted to evaluate its safety and efficacy.