LRallybio Gets Green Light for Phase 2 Study of RLYB212 in High-Risk Pregnant Women
Rallybio Corporation, a biotechnology firm in the clinical development phase focused on converting scientific innovations into groundbreaking treatments for individuals experiencing severe rare conditions, has declared that it received approval for its clinical trial applications concerning a Phase 2 study of RLYB212. This trial targets pregnant women who are at an elevated risk of HPA-1a alloimmunization as well as fetal and neonatal alloimmune thrombocytopenia.
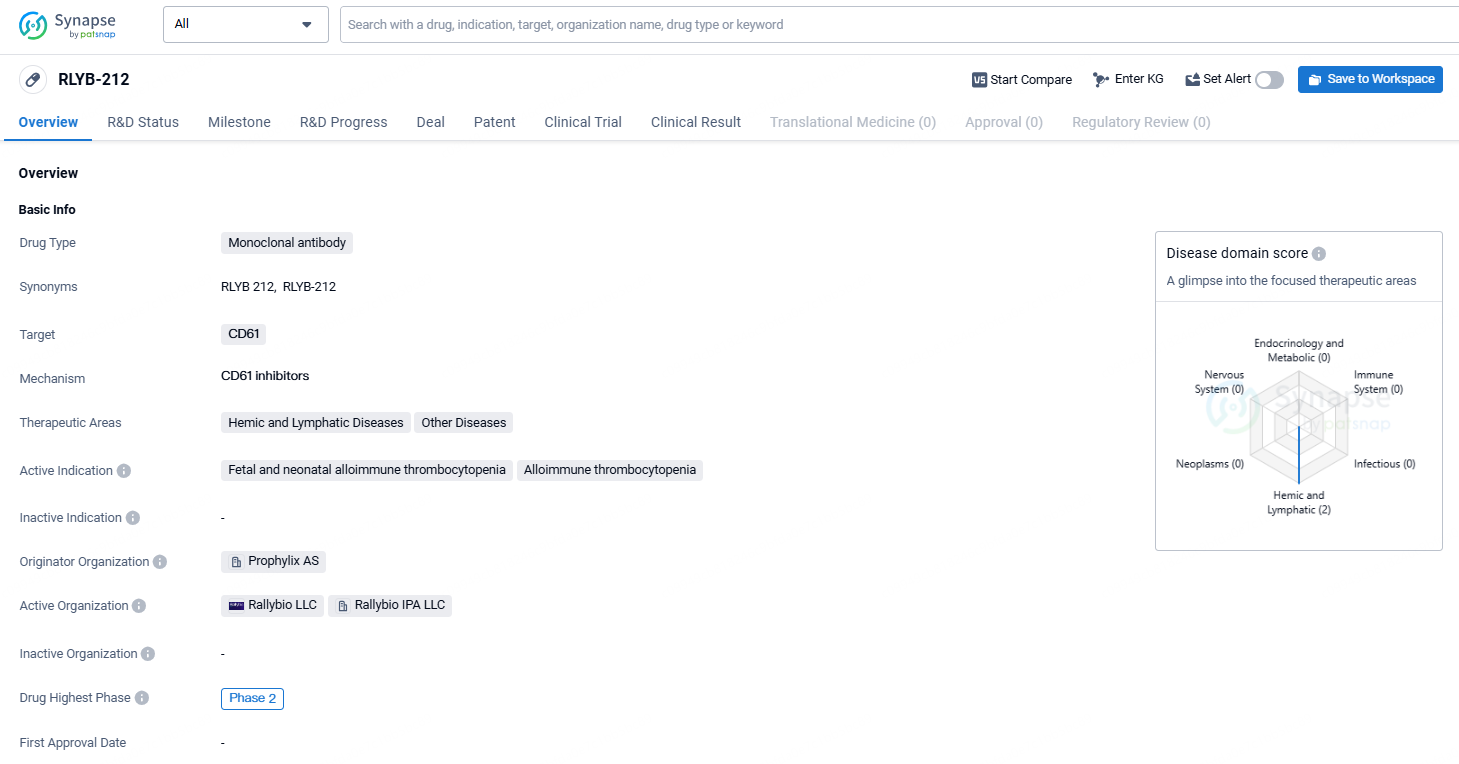
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Following the endorsements from the European Medicines Agency and the UK's Medicines and Healthcare products Regulatory Agency, Rallybio is set to commence the activation of Phase 2 clinical trial sites and anticipates beginning the participant screening process in the last quarter of 2024.
"Achieving these Clinical Trial Application approvals from European regulatory bodies to advance RLYB212 into Phase 2 represents a major milestone. These endorsements reflect the commitment and creativity of our team and collaborators as we progress with this pioneering initiative aimed at preventing maternal alloimmunization and Fetal and Neonatal Alloimmune Thrombocytopenia (FNAIT)," stated Stephen Uden, M.D., the CEO of Rallybio.
"We are in the process of activating clinical sites and expect to start screening this quarter, which signifies another crucial advancement toward fulfilling our goal of preventing FNAIT and mitigating its severe consequences," Uden further expressed.
The single-arm Phase 2 trial focused on dose confirmation will take place across Belgium, the Netherlands, Norway, Sweden, and the UK. It is intended to evaluate the pharmacokinetics and safety profile of RLYB212, a monoclonal anti-HPA-1a antibody, in eight pregnant women identified to be at elevated risk of HPA-1a alloimmunization and FNAIT.
Additional aims involve examining pregnancy outcomes, neonatal/infant results, and the emergence of HPA-1a alloimmunization. The subcutaneous administration of RLYB212 will commence at Gestational Week 16 and will continue at four-week intervals until delivery.
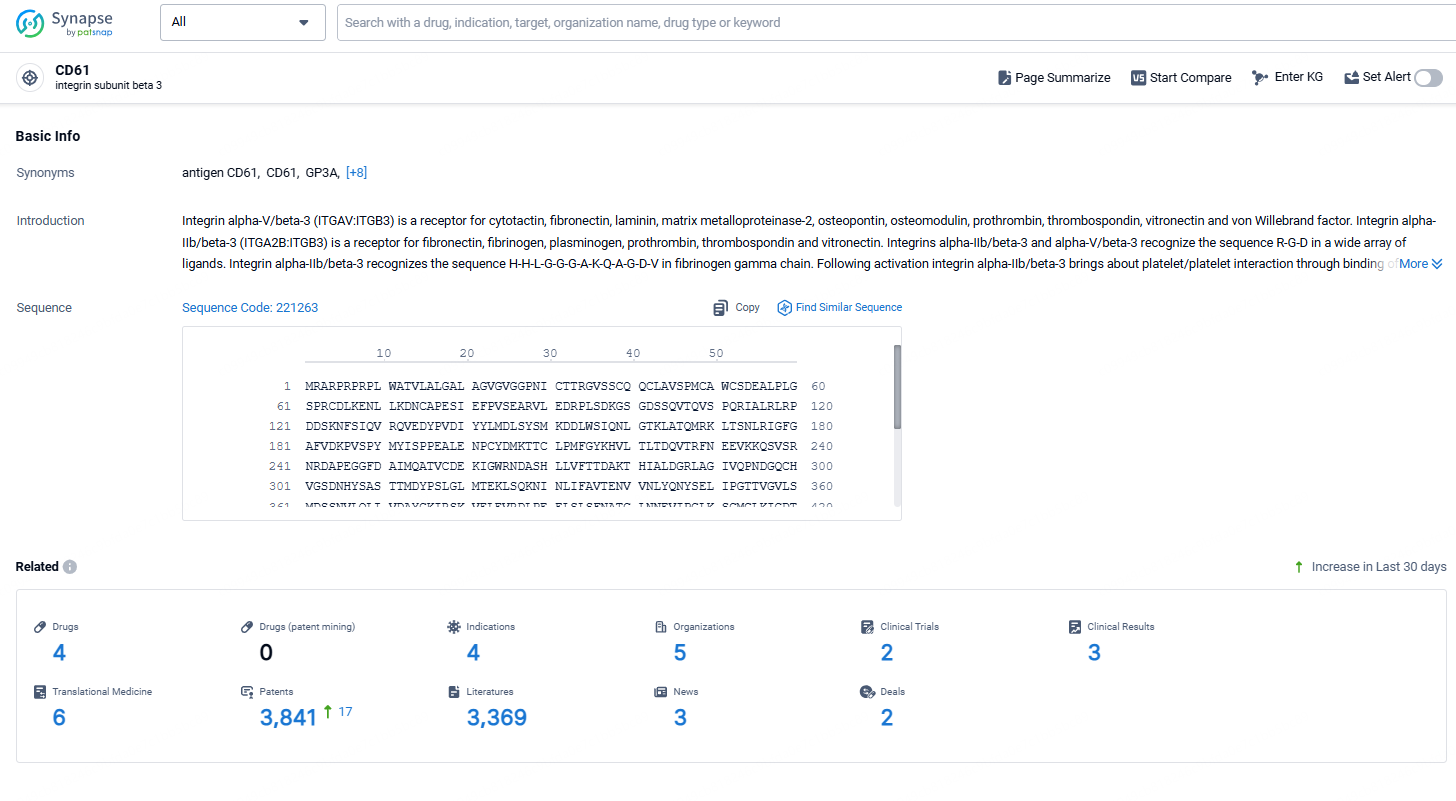
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 31, 2024, there are 4 investigational drugs for the CD61 target, including 4 indications,5 R&D institutions involved, with related clinical trials reaching 2, and as many as 3369 patents.
RLYB-212 is a monoclonal antibody drug developed by Prophylix AS, which targets the CD61 protein and is being developed for the treatment of immune-mediated thrombocytopenia, with a focus on fetal and neonatal alloimmune thrombocytopenia. As it has reached Phase 2 in its development, it shows promise as a potential treatment option for these conditions, although further research is needed to fully establish its clinical utility.