Marea Therapeutics Kicks Off with $190M to Speed Up Innovative Cardiometabolic Drugs
Marea Therapeutics, a biotechnology enterprise focused on clinical-stage developments and backed by Third Rock Ventures, is dedicated to creating innovative treatments for cardiometabolic disorders. The company has been introduced into the market with a total of $190 million secured through both Series A and B funding rounds.
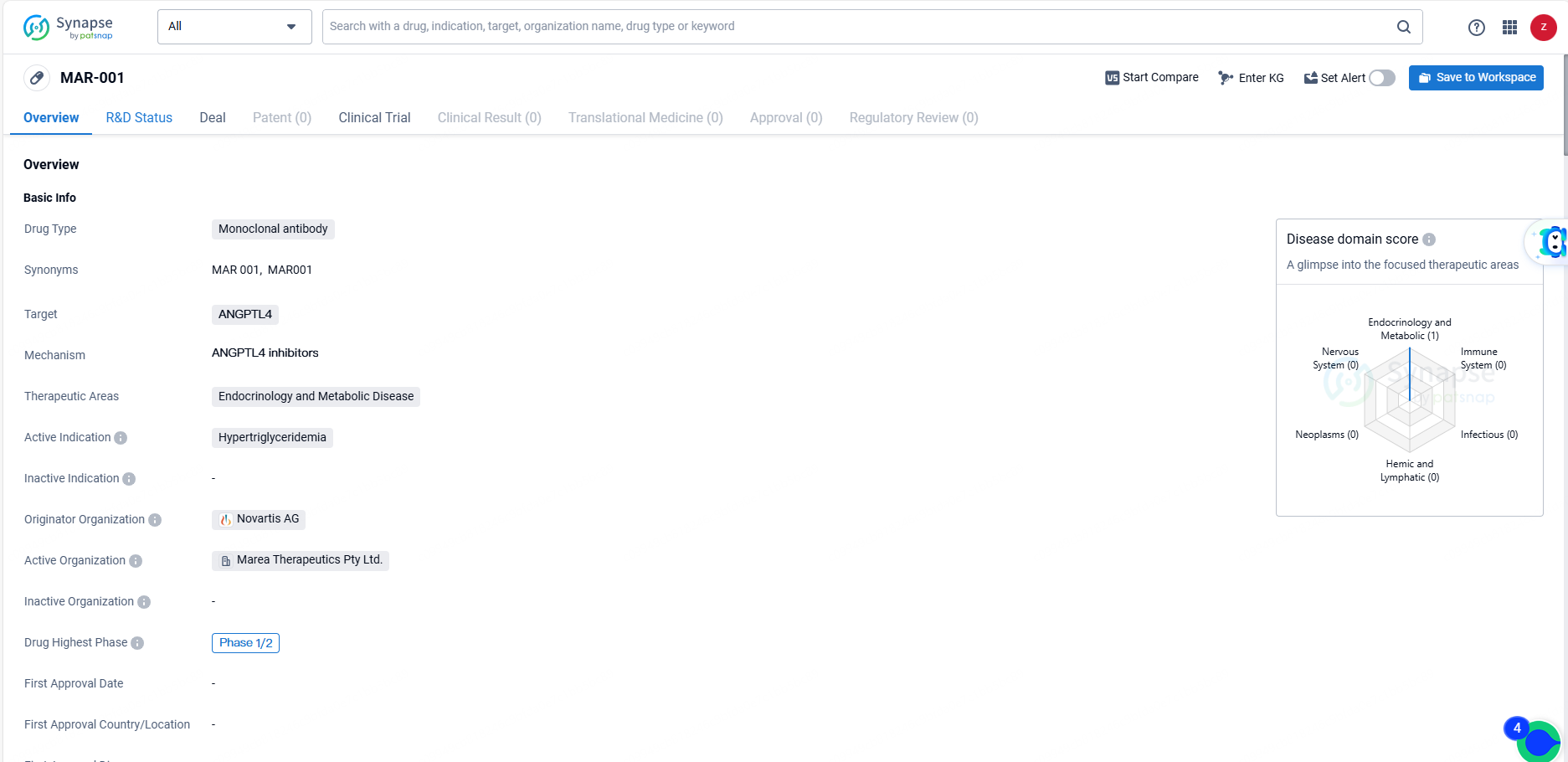
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 The Series A funding round was spearheaded by Third Rock Ventures, while Sofinnova Investments and co-led by Forbion, Perceptive Xontogeny Venture Fund, and venBio led the Series B round. Additional participation came from Alpha Wave Global, Omega Funds, Surveyor Capital, and initial investor Third Rock Ventures. This funding will support the company's Phase 2 development of MAR001, as well as other ongoing projects in the pipeline.
The Series A funding round was spearheaded by Third Rock Ventures, while Sofinnova Investments and co-led by Forbion, Perceptive Xontogeny Venture Fund, and venBio led the Series B round. Additional participation came from Alpha Wave Global, Omega Funds, Surveyor Capital, and initial investor Third Rock Ventures. This funding will support the company's Phase 2 development of MAR001, as well as other ongoing projects in the pipeline.
“Marea is committed to revolutionizing the treatment of cardiometabolic diseases by leveraging the power of large-scale human genetics and specialized knowledge in adipose tissue function to target genetically validated factors that play a central, yet often neglected role in cardiometabolic disease,” said Josh Lehrer, M.D., M.Phil., FACC, CEO of Marea. “Our approach holds potential promise for patients who persistently face high risks despite existing therapies.”
“With initial clinical validation, premier scientific founders and investors, and a seasoned board and leadership team, Marea is on the path to becoming a leading company in the cardiometabolic disease space,” commented Jeffrey Tong, Ph.D., board member and partner at Third Rock Ventures. “Our objective is to propel a new wave of drugs, including MAR001, to tackle critical, unaddressed drivers of cardiometabolic diseases, and offer significant new therapeutic options to countless patients.”
Marea’s primary program, MAR001, is an antibody that specifically targets ANGPTL4, a protein predominantly found in adipose tissue. Inhibiting ANGPTL4 enhances activity of adipose tissue lipoprotein lipase (LPL), aiming to lower remnant cholesterol, boost adipose tissue and metabolic function, and decrease cardiovascular events. Triglyceride-rich lipoproteins, carriers of remnant cholesterol, are extremely atherogenic and cause cardiovascular issues independently of typical risk factors like LDL cholesterol, diabetes, or obesity. Currently, there are no specific therapies available to reduce remnant cholesterol and improve metabolic function.
“A single dose of MAR001 in a Phase 1 trial significantly reduced remnant cholesterol levels and improved metabolic biomarkers. We are highly enthusiastic about the potential of this compound,” said Maha Katabi, Ph.D., general partner at Sofinnova Investments. “Driven by experts in genetics and cardiometabolic diseases, Marea is well-placed to advance MAR001 and other pipeline projects, heralding a new era in cardiovascular care.”
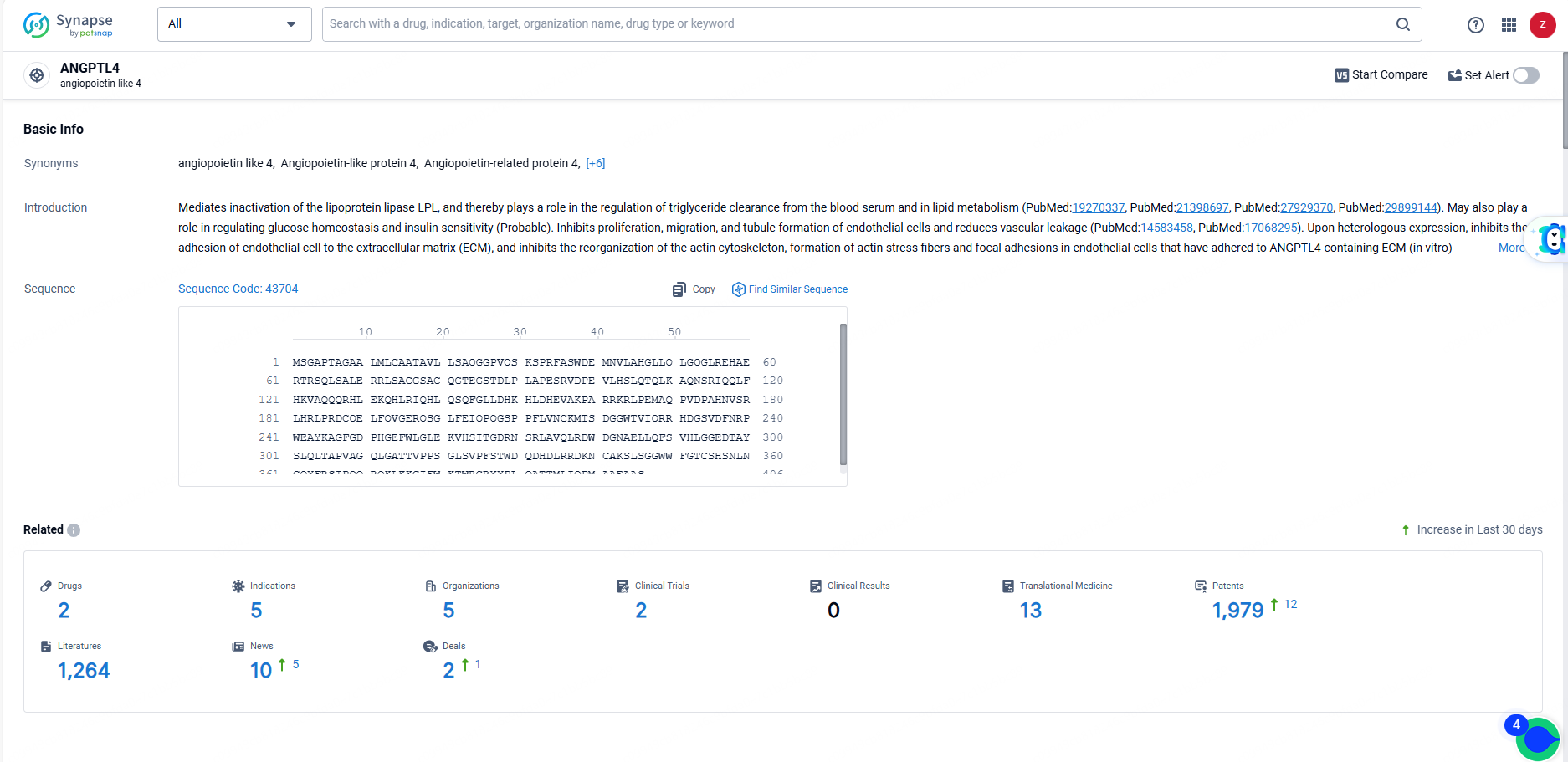
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 23, 2024, there are 2 investigational drugs for the ANGPTL4 target, including 5 indications, 5 R&D institutions involved, with related clinical trials reaching 2, and as many as 1979 patents.
MAR-001 is a monoclonal antibody drug targeting ANGPTL4, with a focus on treating hypertriglyceridemia in the therapeutic areas of endocrinology and metabolic disease. It is currently in Phase 1/2 of global development, and its originator organization is Novartis AG. The drug shows potential in addressing the unmet medical need for effective treatments for hypertriglyceridemia.