Monopar begins Phase 1 study of MNPR-101-Zr radioactive drug in individuals with late-stage cancer
Monopar Therapeutics Inc., an enterprise in the clinical-phase biopharmaceutical domain committed to crafting groundbreaking therapies for individuals with cancer, revealed the initiation and patient enrollment for its pioneering Phase 1 dosimetry clinical study. This trial revolves around the unique radiopharmaceutical diagnostic molecule MNPR-101-Zr, which is a chemical conjugate of MNPR-101 with the isotope zirconium-89. MNPR-101 is an antibody designed to target and bind to the urokinase plasminogen activator receptor (uPAR), ubiquitously found across a spectrum of malignant forms, including tumors of the pancreas, breast, colorectum, and bladder.
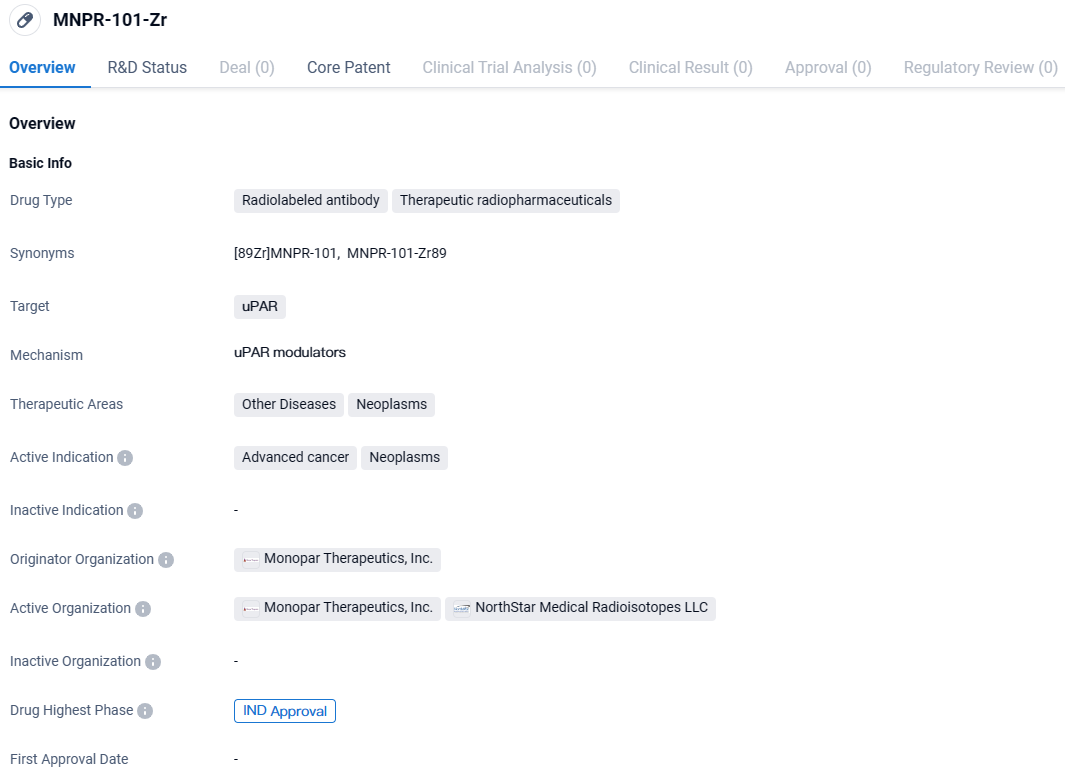
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Recruitment has commenced at the Melbourne Theranostic Innovation Centre (MTIC) in Australia for the study, under the guidance of globally esteemed medical professional and radiopharma innovator, Professor Rodney Hicks. The Centre is utilizing a highly sensitive and advanced Siemens Biograph Vision Quadra, one of the top-tier total-body PET/CT scanners globally, to assess the efficacy of MNPR-101-Zr in homing in on tumors within patients suffering from late-stage cancers.
The initial phase clinical trial involves a safety and dosimetry assessment of MNPR-101-Zr, which will involve a cohort of up to 12 participants diagnosed with advanced forms of cancer. Existing preclinical findings have demonstrated precise and long-lasting localization of MNPR-101-Zr in tumors developed from human cancerous tissues in animal models.
In addition, Monopar Therapeutics has disclosed compelling preclinical findings that suggest strong and persistent cancer-fighting effects of MNPR-101 when conjugated with therapeutic radioisotopes. Should the preliminary clinical trial evidence point toward promising results for tumor uptake, distribution throughout the body, and safety profile for MNPR-101-Zr, the Company is considering either broadening the scope of the present study or commencing a subsequent trial to evaluate the efficacy of MNPR-101 when attached to a cancer-treating radioisotope such as Actinium-225 in individuals with progressive stages of cancer.
Chandler Robinson, MD, the CEO of Monopar Therapeutics, expressed enthusiasm for the commencement of the clinical study, highlighting the preclinical success seen against challenging cancers inclusive of pancreatic and triple-negative breast cancer. He noted Monopar’s radiopharmacy initiative has shown a noteworthy potential in selectively engaging and eliminating tumors that exhibit uPAR. The team at Monopar anticipates the forthcoming human trial data on biodistribution and dosimetry in the context of advanced cancers.
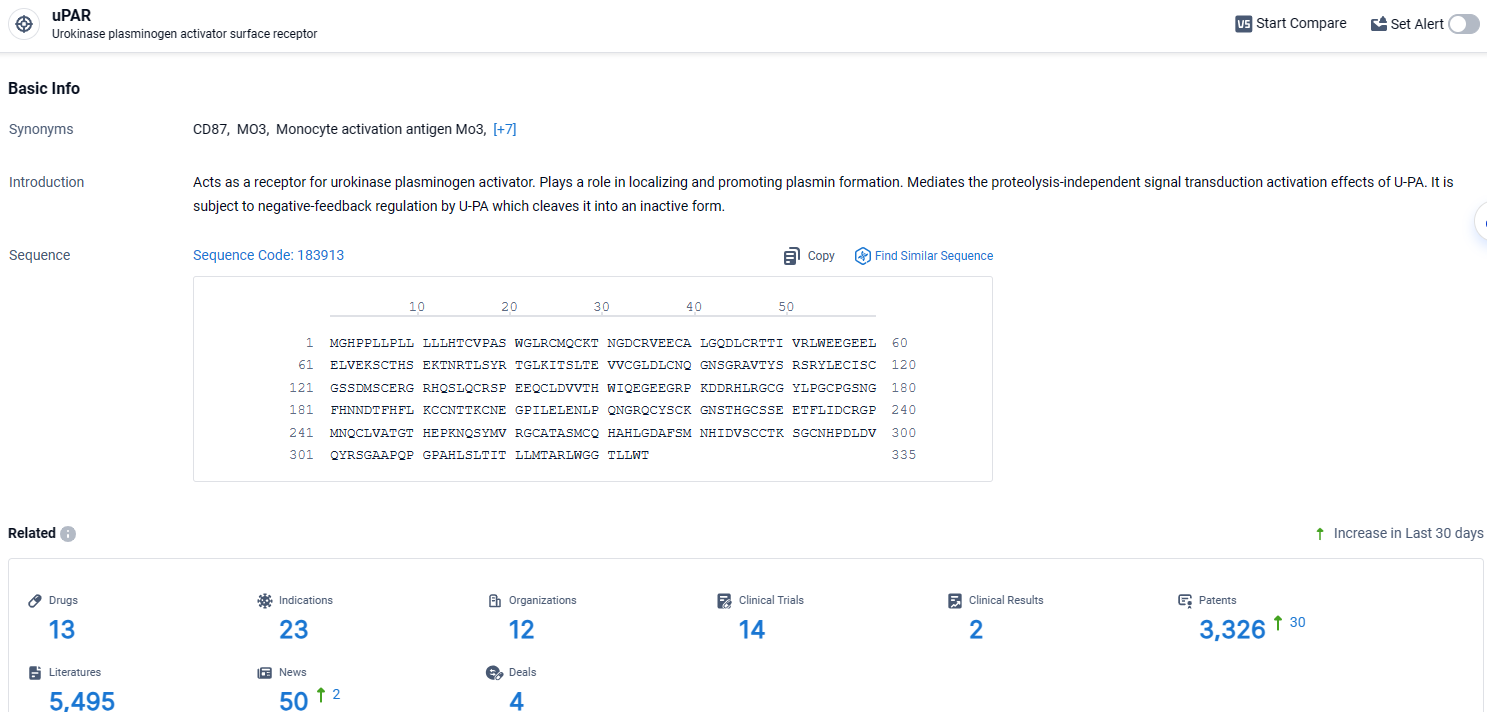
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 11, 2024, there are 13 investigational drugs for the uPAR target, including 23 indications, 12 R&D institutions involved, with related clinical trials reaching 14, and as many as 3326 patents.
MNPR-101-Zr is a radiolabeled antibody therapeutic radiopharmaceutical that targets uPAR. It is being developed by Monopar Therapeutics, Inc. for the treatment of advanced cancer and neoplasms. The drug has reached the IND approval stage, indicating its potential for further clinical development.