Neurocrine Initiates Phase I Trial of VMAT2 Inhibitor NBI-1065890
Neurocrine Biosciences, Inc. has launched a Phase 1 clinical trial to examine the safety profile, acceptability, absorption, distribution, and effect mechanisms of the trial drug NBI-1065890 in a group of healthy adults. The substance NBI-1065890 under examination is a targeted oral therapy that acts as a selective blocker of VMAT2, and it may offer therapeutic benefits for a range of neurologic and mental health disorders.
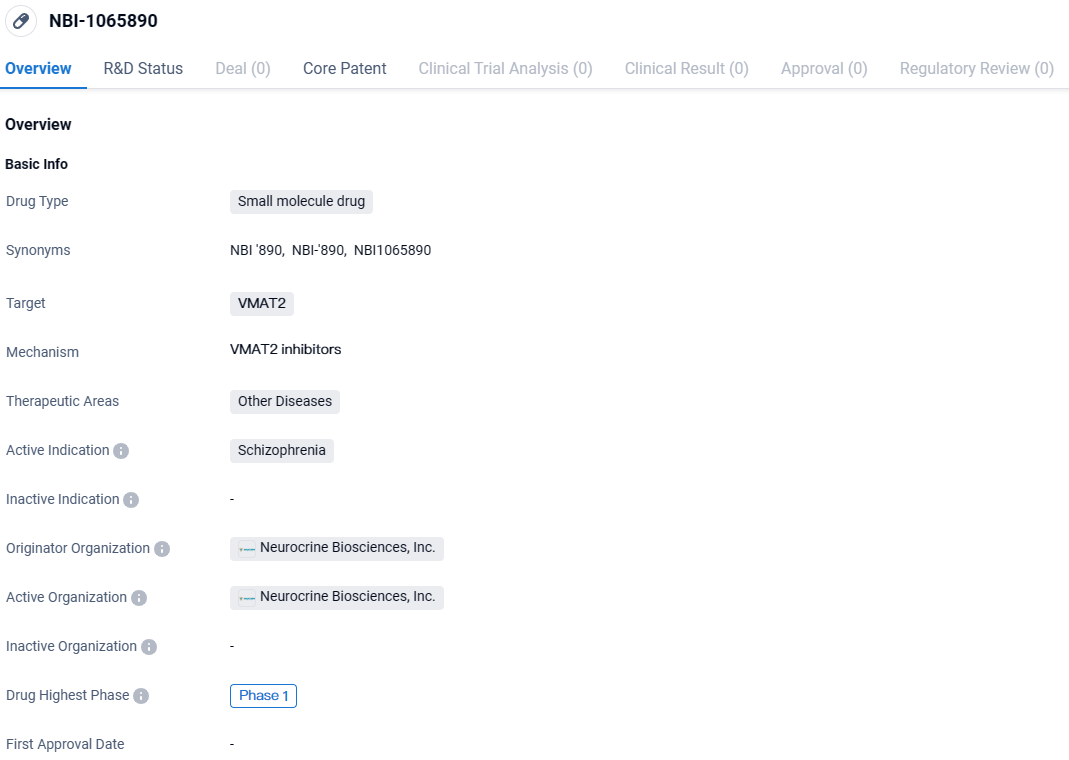
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Neurocrine Biosciences possesses profound expertise and a track record in the realm of VMAT2 inhibition, as indicated by the creation and progression of valbenazine as a remedy for the involuntary movements related to tardive dyskinesia as well as Huntington’s disease-associated chorea, declared Eiry W. Roberts, Chief Medical Officer at the organization.
Dr. Roberts further articulated enthusiasm for the advancement of a novel, proprietary, excessively active, and orally administered selective VMAT2 inhibitor to clinical trials, with aspirations to offer a unique therapeutic option for various neurological and psychiatric disorders.
Compounds that impede VMAT2 have been demonstrated in clinical settings to be efficacious in managing disorders characterized by excessive motor activity, and are significant in the presynaptic regulation of dopamine. In 2017, Neurocrine successfully gained authorization from the U.S. Food and Drug Administration for valbenazine as the premier medication fashioned specifically for the management of tardive dyskinesia, substantiating the company’s leadership in selective VMAT2 inhibition. Furthering its achievements, the firm acquired FDA endorsement for valbenazine to address chorea manifestations of Huntington’s disease in 2023.
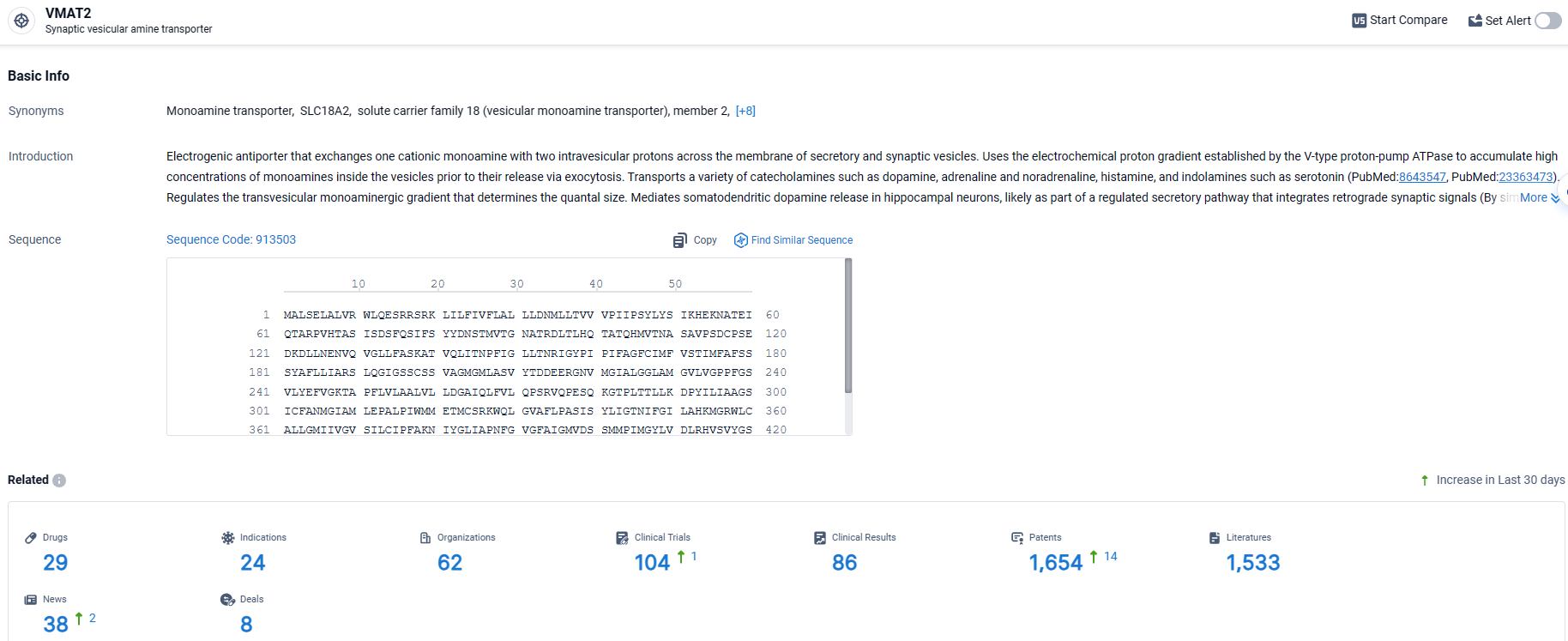
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 1, 2024, there are 29 investigational drugs for the VMAT2 target, including 24 indications, 62 R&D institutions involved, with related clinical trials reaching 104, and as many as 1654 patents.
NBI-1065890 targets VMAT2 and is being developed for the treatment of schizophrenia. Currently in Phase 1, this drug holds promise for potentially addressing the unmet medical needs of patients with this debilitating mental diseases.