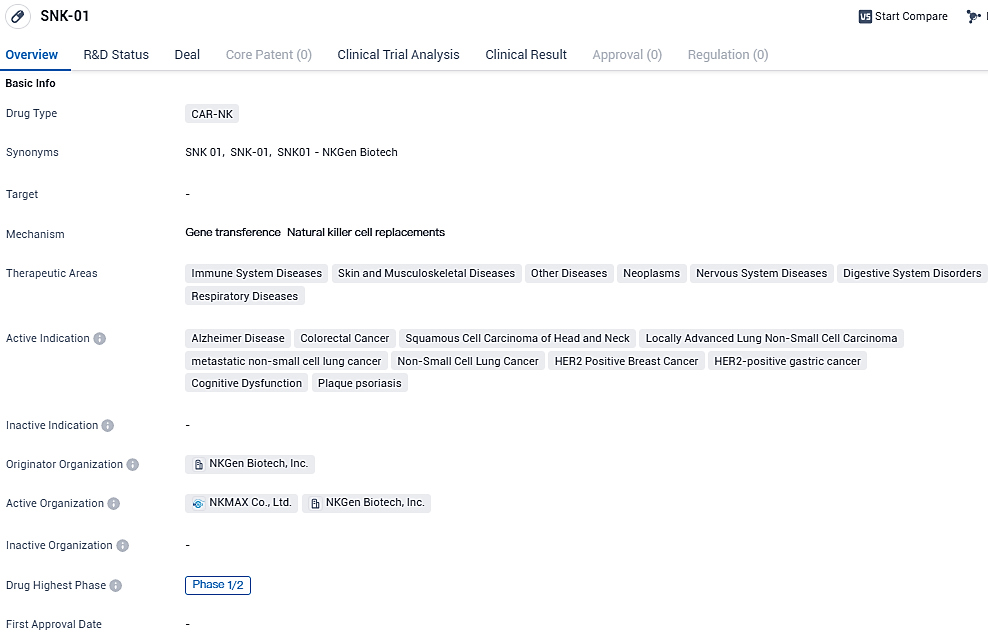
NKGen Biotech, Inc. has begun treating the first participant in a Phase 1/2a Study with its SNK01 for moderate Alzheimer's Disease
NKGen Biotech, Inc.,, a biotech company engaged in clinical-phase research, is dedicated to pioneering the creation and market launch of cutting-edge therapeutic solutions involving autologous, allogeneic, and CAR-NK natural killer cells. The company has recently initiated treatment in its early to mid-stage clinical study with the administration of SNK01 to the initial participant.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
SNK01 is a specialized therapy utilizing cryogenically preserved autologous NK cells that have not been genetically altered, featuring augmented cytotoxic capabilities and increased expression of activating receptors. The ongoing clinical trial, in its Phase 1/2a stage, is focusing on assessing the safety and the ease of administering this therapeutic approach to individuals with moderate Alzheimer's Disease (AD).
The initial stage, Phase 1, is a non-blinded safety assessment aimed at identifying the maximum dosage patients can tolerate without adverse effects, which will also inform the dosing recommendation for Phase 2. Subsequently, Phase 2 is conceived as a controlled, double-blind study aiming to appraise both the safety profile and the therapeutic efficacy of SNK01 for patients grappling with moderate AD.
NKGen Biotech's CEO, Paul Song, M.D., expressed enthusiasm about the commencement of dosing with SNK01 in the current Phase 1/2a study, targeting a significant gap in treatment options for Alzheimer's using their advanced cryopreserved formulation. Dr. Song elaborated on the trial's structure, revealing plans to administer 6 billion cells every 21 days over a 12-month period in a head-to-head comparison against a placebo.
The goal is to achieve a pronounced positive impact on cognitive function and mitigate both protein buildup and neuroinflammation associated with the disease. An interim analysis of the gathered data is slated for the third quarter of 2024.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
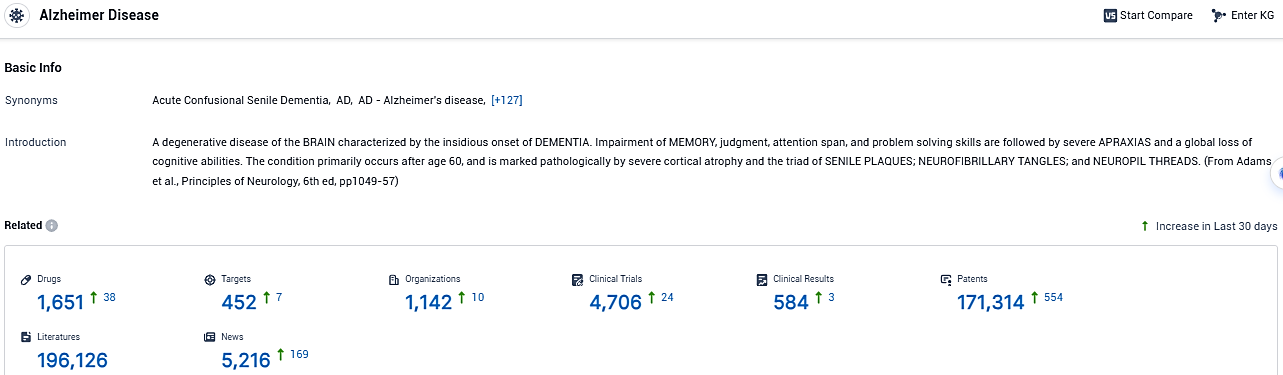
According to the data provided by the Synapse Database, As of January 4, 2024, there are 1651 investigational drugs for the Alzheimer Disease, including 452 targets, 1142 R&D institutions involved, with related clinical trials reaching 4907, and as many as 171314 patents.
SNK01 is a novel cell-based, patient specific ex vivo expanded autologous natural killer cell, immunotherapeutic drug candidate. NKGen Biotech, Inc. is developing SNK01 for the treatment of neurodegenerative disorders and a broad range of cancers.