Nurix Reveals NX-1607, a Novel CBL-B Inhibitor, at ACS Conference
Nurix Therapeutics, Inc., a company specialized in the clinical development phase and dedicated to crafting drugs that modulate specific proteins aimed at addressing cancers and inflammatory conditions, has recently revealed the identification and molecular architecture of NX-1607. The revelation occurred during the initial disclosure segment at the Spring 2024 convention of the American Chemical Society held in New Orleans, LA. Marking a pioneering stride in medicinal advancement, NX-1607 represents the initial therapeutic candidate to suppress CBL-B, thereby embarking on a path towards clinical trials. It stands as a testament to Nurix's innovative capabilities in engaging with previously deemed "undruggable" E3 ligases.
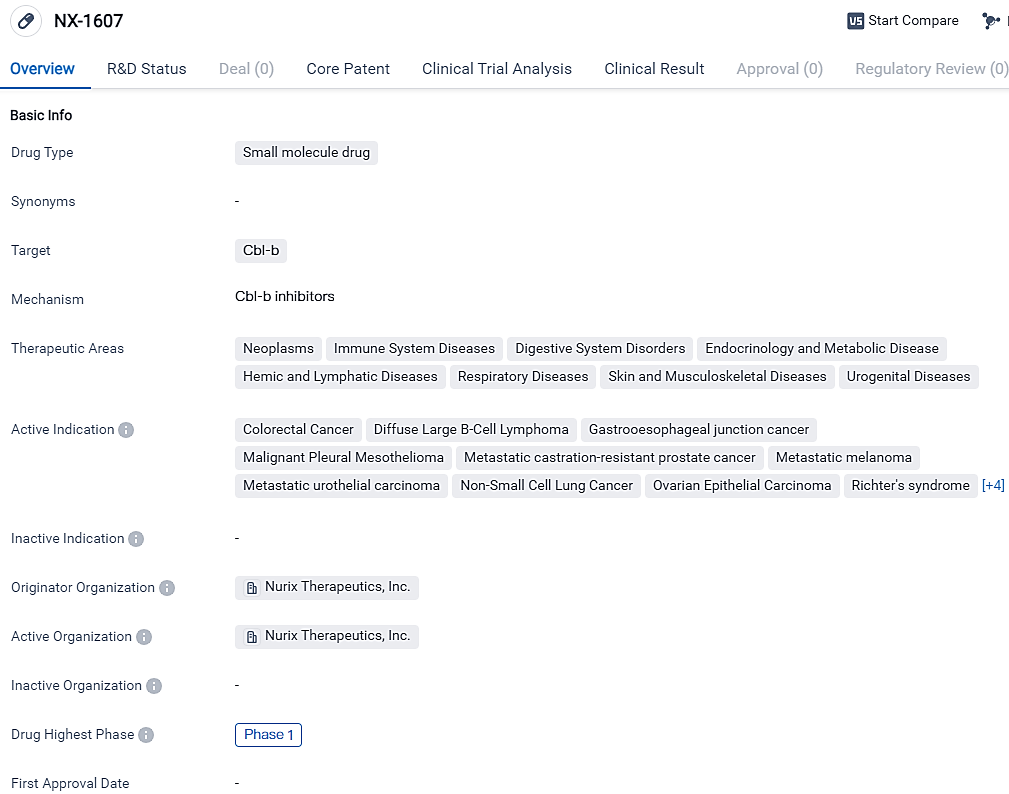
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
During the session at the ACS conference, Nurix revealed its cutting-edge integration of structure-based-drug-design with its unparalleled biochemistry knowledge of E3 ligases, Dr. Gwenn Hansen, Ph.D., the company's chief scientific officer, highlighted. Through its distinctive approach in the realm of drug development, Nurix is capable of crafting pioneering compounds that target E3 ligases for inhibition, such as NX-1607, or exploit these ligases for the precise breakdown of proteins.
In a verbal exposition at the American Chemical Society Spring conference addressing ‘NX-1607: a Novel CBL-B Inhibitor for Immuno-Oncology,’ Nurix unveiled the molecular architecture of NX-1607 along with the chronicle of its conception. As a selective binder within the CBL-B molecule, NX-1607 secures it in a static, non-active shape that amplifies the sensitivity for T-cell activation.
The action model of NX-1607 is distinctive, as CBL-B does not feature the conventional active site seen in enzymes that would typically be targeted for inhibition, demanding an innovative angle for drug formulation. The materials from the talk can be found in the Posters and Presentations segment on Nurix’s scientific resources webpage.
Currently, Nurix is appraising NX-1607 within a novel, first-in-human, multicenter, Phase 1a/1b trial without a placebo, investigating both the dose-titration and the extension phases to assess the compound's safety profile, tolerability range, pharmacological behavior and dynamics, along with its initial anti-cancer efficacy in patients grappling with severe cancers, encompassing various solid tumors and lymphomas.
The study comprises not only a solo regimen but also a combined treatment with paclitaxel. By 2024, Nurix anticipates disseminating findings from the initial dose-building phase of NX-1607's trial and establishing an appropriate dose for subsequent enlargement of the Phase 1b participant group.
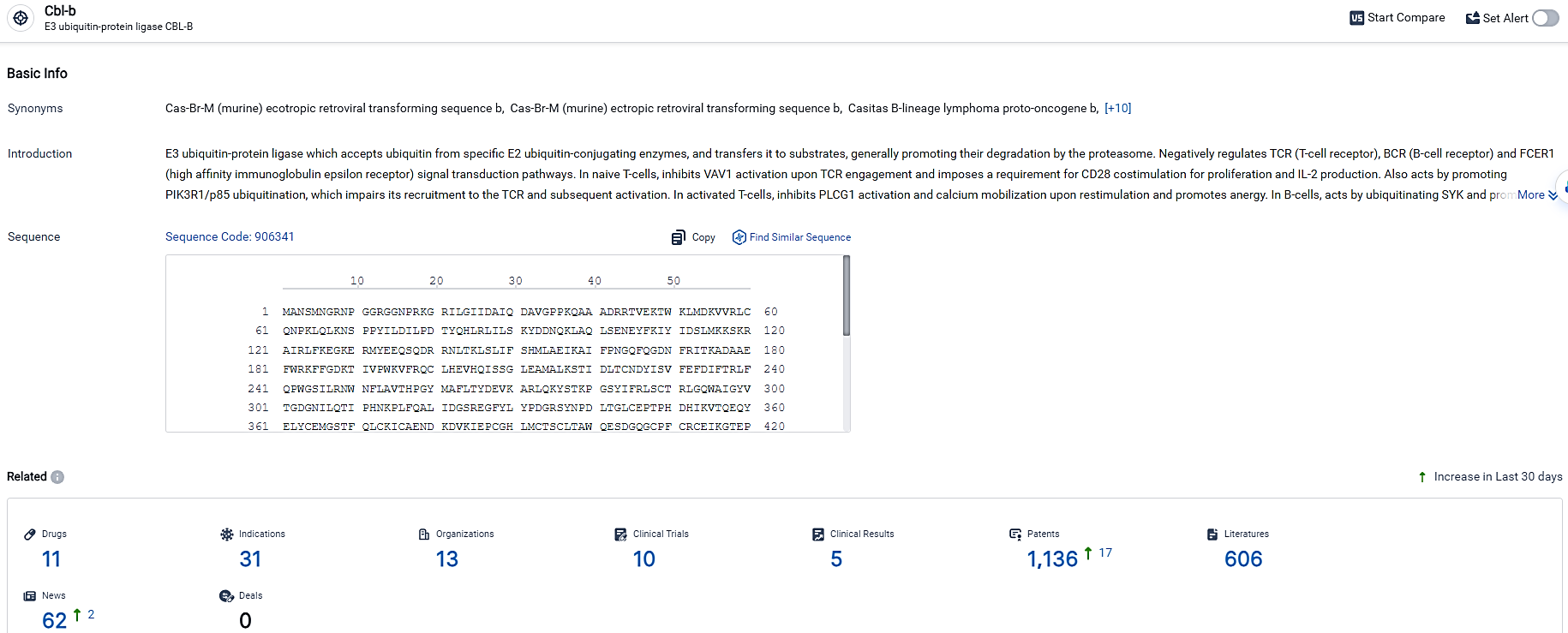
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 22 2024, there are 11 investigational drugs for the CBL-B target, including 31 indications, 13 R&D institutions involved, with related clinical trials reaching 10, and as many as 1136 patents.
NX-1607 is an investigational drug with a broad range of potential therapeutic applications in various disease areas. Its current highest phase of development is Phase 1, indicating that it is still in the early stages of clinical testing. Further research and clinical trials will be necessary to determine the safety and efficacy of NX-1607 in treating the indicated diseases.