Nurix Therapeutics Presents Early Data on Autoimmune Programs NX-5948 and GS-6791 at ACR 2024
Nurix Therapeutics, Inc. (Nasdaq: NRIX), a biopharmaceutical firm in clinical development focused on targeted protein modulation therapies for cancer and inflammatory conditions, has announced the disclosure of preclinical findings, encompassing mechanisms of action and pertinent disease models, from two of its drug development programs: NX-5948 and GS-6791. NX-5948 is Nurix’s unique, orally administered Bruton’s tyrosine kinase (BTK) degrader that penetrates the brain, currently being investigated for the treatment of inflammatory and autoimmune disorders, alongside an ongoing Phase 1b trial in patients with B-cell tumors. GS-6791 is a selective, orally bioavailable degrader of interleukin-1 receptor-associated kinase 4 (IRAK4) that is being developed in partnership with Gilead Sciences for the potential management of rheumatoid arthritis and various inflammatory diseases. These findings were showcased through two posters at ACR Convergence 2024, the yearly conference of the American College of Rheumatology (ACR), taking place from November 14-19, 2024, in Washington, D.C.
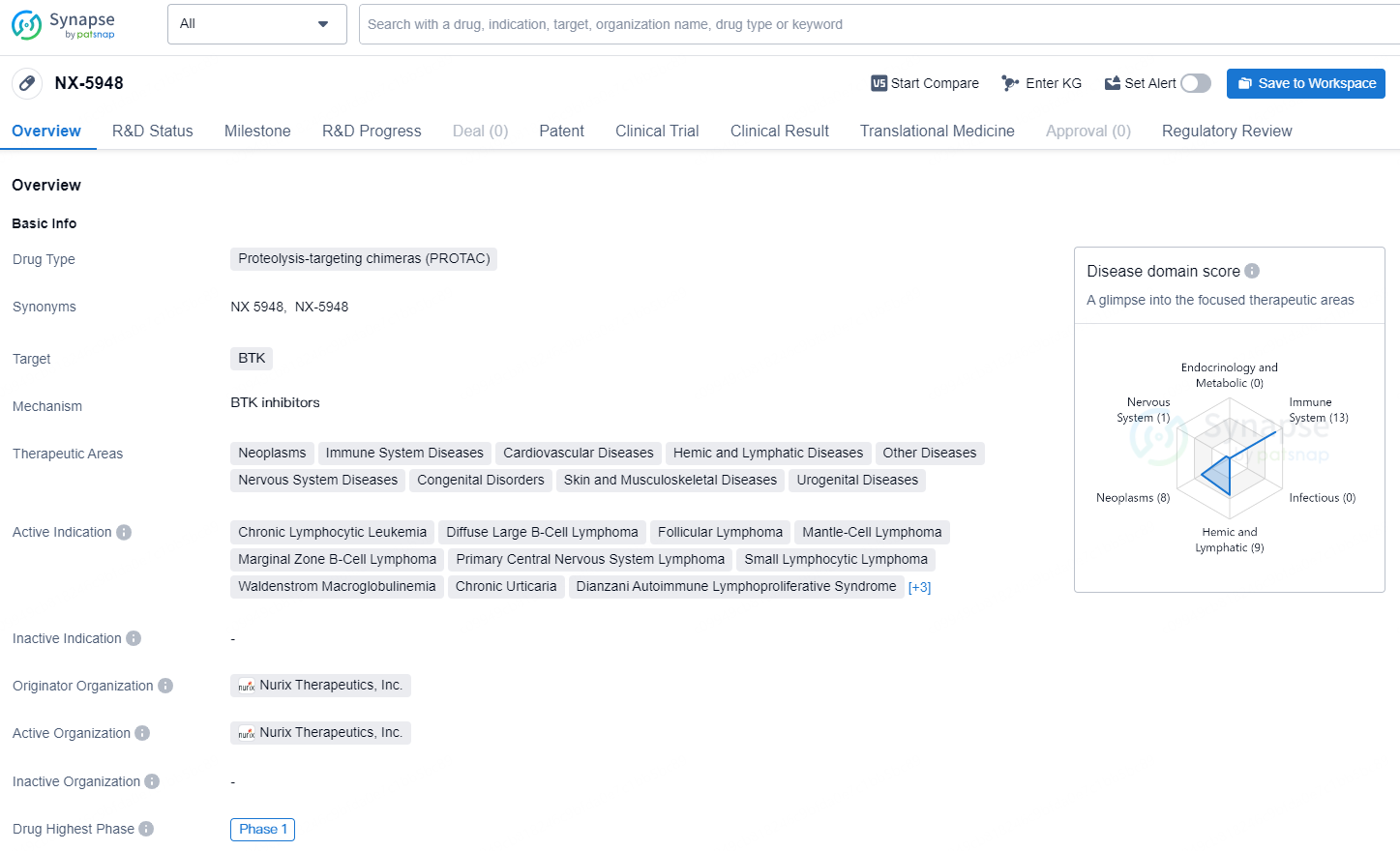
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The preclinical findings shared at ACR Convergence highlight the remarkable promise of our targeted protein degradation approach in comparison to kinase inhibition concerning BTK and IRAK4, which are essential targets in the realm of inflammatory and autoimmune disorders, thereby justifying the further progress of these drug candidates into clinical trials," stated Arthur T. Sands, M.D., Ph.D., the president and CEO of Nurix. "These initiatives demonstrate the effectiveness of Nurix’s DELigase platform in developing powerful top-tier degrader drug candidates that could offer enhanced efficacy across various inflammatory conditions."
BTK is involved in the signaling pathways activated by the B cell receptor (BCR), toll-like receptors (TLRs), and Fc receptors (FcRs), positioning it as a promising treatment target for antibody-driven autoimmune and inflammatory illnesses. It exhibits both kinase and scaffold activities that are crucial for its role. Inhibiting BTK may lower the generation of new antibodies and lessen inflammation caused by pre-existing antibodies, addressing pivotal issues in inflammatory and autoimmune conditions.
According to a presentation titled: "NX-5948, a Clinical-Stage BTK Degrader, Achieves Deep Suppression of BCR, TLR, and FcR Signaling in Immune Cells and Demonstrates Efficacy in Preclinical Models of Arthritis and Other Inflammatory Diseases," data indicate the advantages of the BTK degrader NX-5948, which matches or surpasses BTK inhibition across various mechanistic investigations and models of inflammatory disorders. In primary B cells, NX-5948 facilitates swift BTK degradation and more effectively inhibits proximal BCR signaling and triggers BCR- and TLR-mediated B cell activation compared to existing BTK inhibitors under development.
In a model of established collagen-induced arthritis, oral dosing of NX-5948 yields comparative or improved clinical score enhancements and a more pronounced reduction in plasma cell counts versus BTK inhibitors. NX-5948 also shows effectiveness across numerous other inflammatory disease models, including antibody-induced glomerulonephritis (representing lupus nephritis), autoimmune lymphoproliferative syndrome (ALPS, another lupus-like disease model), passive cutaneous anaphylaxis (an allergenic response model encompassing chronic spontaneous urticaria), and experimental autoimmune encephalitis (an analog for multiple sclerosis).
IRAK4 is vital for TLR- and interleukin-1 receptor family (IL-1R) signaling, which triggers inflammatory responses. Similar to BTK, IRAK4 possesses both kinase and scaffold roles, with the latter being particularly important in mediating IL-1 and TLR signaling across various cell types. GS-6791, an IRAK4-targeted protein degrader, offers a distinct mechanism of action as opposed to merely inhibiting kinase activity.
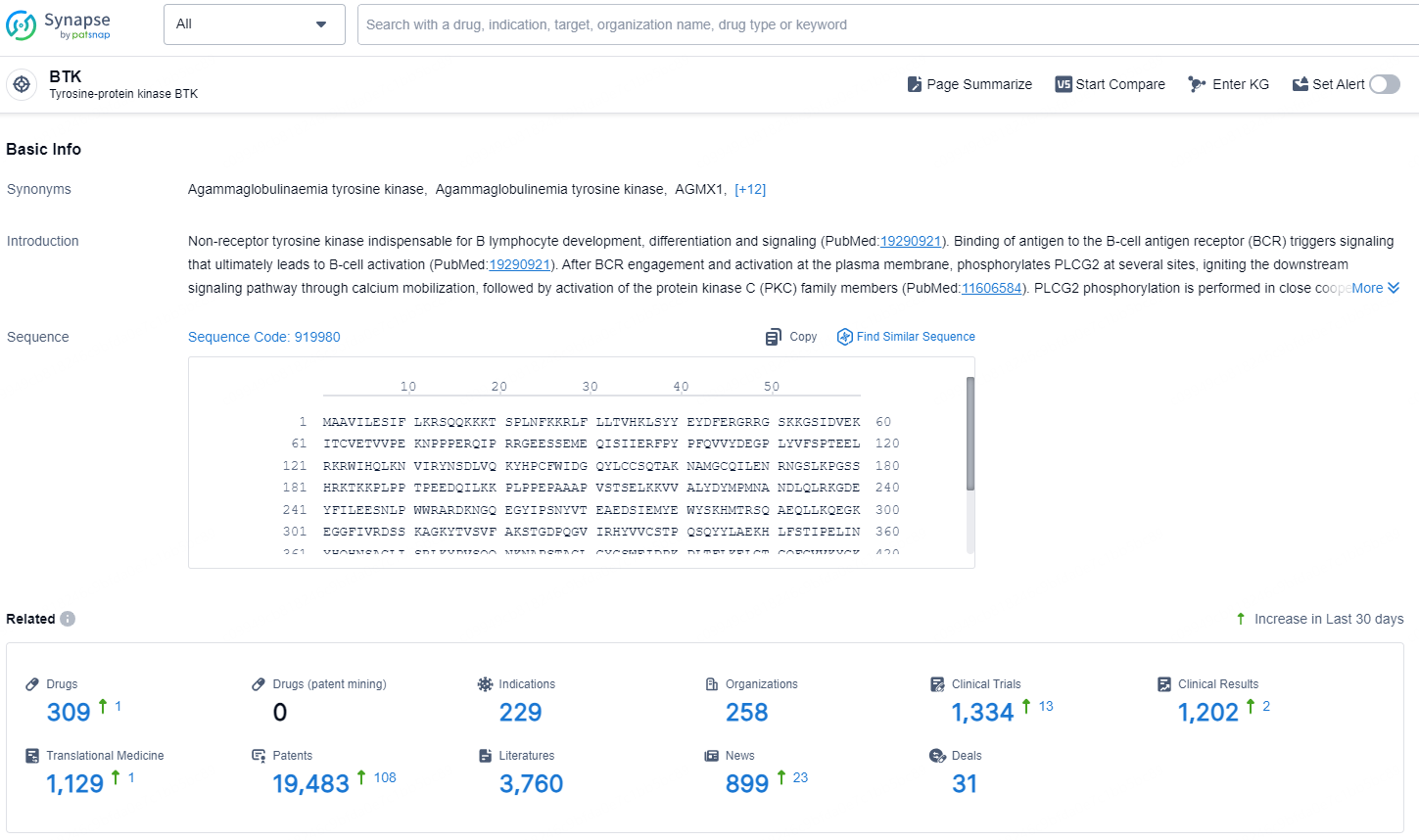
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 20, 2024, there are 309 investigational drugs for the BTK target, including 229 indications, 258 R&D institutions involved, with related clinical trials reaching 1334, and as many as 19483 patents.
NX-5948 is a proteolysis-targeting chimera (PROTAC) drug designed to target the BTK protein. The drug is being developed by Nurix Therapeutics, Inc. and has reached the highest global phase of Phase 1. The drug is designated as a Fast Track drug, indicating its potential to address unmet medical needs.