OncoC4 Initiates Phase 1 Trial of SIGLEC10 Inhibitor ONC-841 for Solid Tumors
OncoC4, Inc. (“OncoC4”), a biopharmaceutical company at the late-stage of development focusing on innovative treatments for cancer and immunological disorders, has disclosed that the initial patient has been administered a dose in the pioneering human Phase 1 ONC-841-002 trial (NCT06352359). This study is assessing ONC-841, a pioneering SIGLEC10 blocking antibody, as a potential therapy for solid tumors.
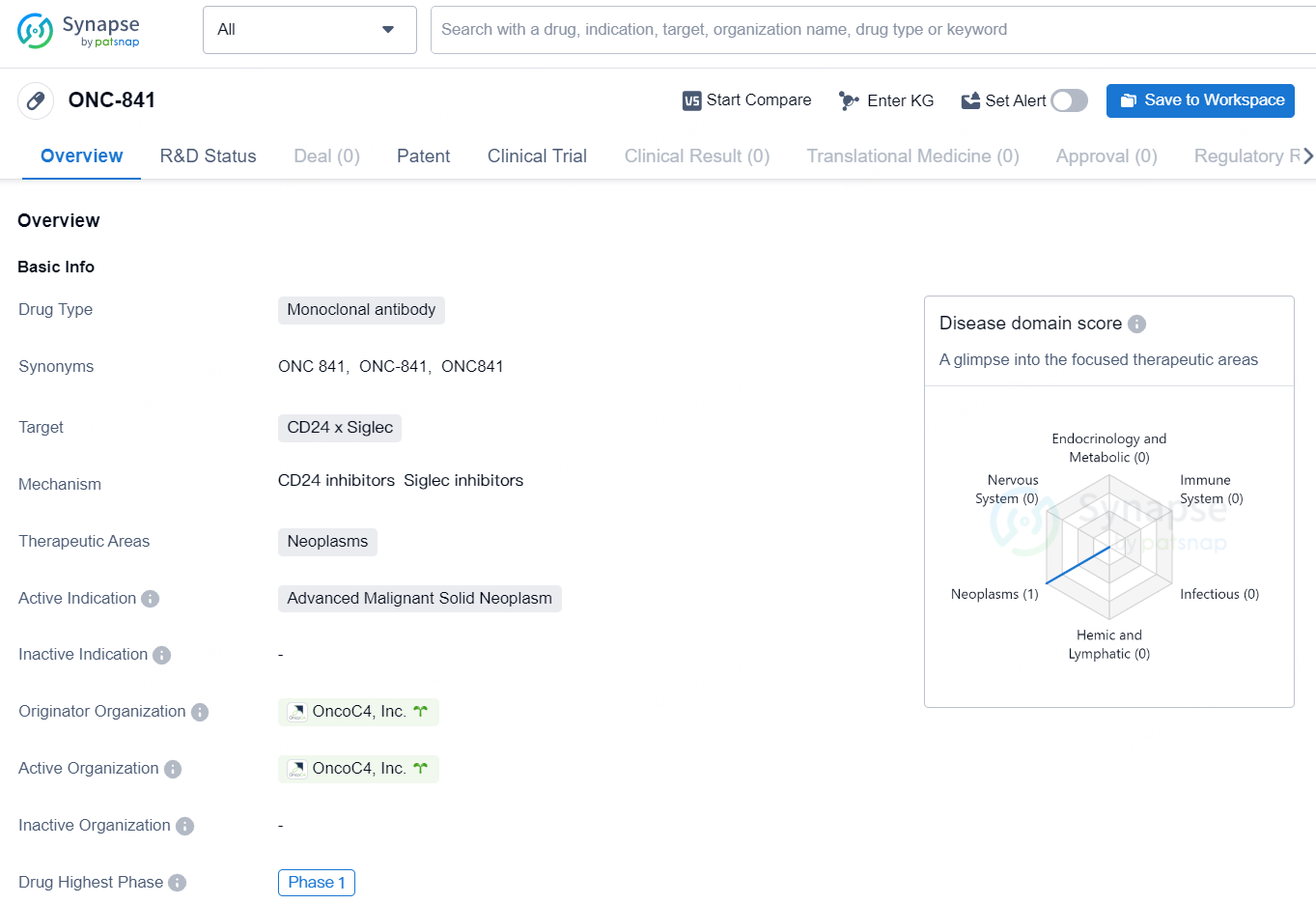
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“Administering the initial dose to the first participant in this inaugural human trial for ONC-841 represents a significant milestone for our SIGLEC initiative, which is anticipated to have potential in treating various cancers,” expressed Dr. Yang Liu, Co-Founder, CEO, and CSO of OncoC4. “ONC-841 aims to inhibit the novel SIGLEC10 immune checkpoint, countering the tumor's ability to evade the immune system by activating anti-tumor immune responses within the tumor microenvironment. We are thrilled about the wide-ranging therapeutic prospects of ONC-841 and eagerly await the preliminary Phase 1 data expected in 2025."
ONC-841-002 is a Phase 1 open-label, dose-escalation study evaluating the safety, efficacy, and pharmacokinetics of ONC-841 as a monotherapy in patients with advanced or metastatic solid tumors. The study will examine seven dose levels of intravenous ONC-841 administered once every 4 weeks at multiple clinical sites across the United States. The trial’s principal investigator is Dr. Tianhong Li from the University of California, Davis Comprehensive Cancer Center. OncoC4 enrolled its first patient under the supervision of site investigator Dr. John Hamm from Norton Cancer Institute in Louisville, Kentucky. Preliminary data on the safety, pharmacokinetics, and clinical activity of ONC-841 monotherapy is anticipated in 2025.
Dr. Hamm commented, “Being part of this journey is very exciting. As oncologists, we are aware of the need for better solutions, and through clinical trials like this, particularly in the last five years, we have observed rapid advancements in cancer care and treatment.”
Developed internally, ONC-841 is a humanized antagonist anti-SIGLEC10 monoclonal antibody. To our knowledge, it is pioneering as the first SIGLEC10 antagonist to enter clinical trials. Siglec10 is an inhibitory receptor gene widely found in tumor-infiltrating immune cells and is crucial in tumor immune evasion through its interaction with CD24 on tumor cells. ONC-841 disrupts this interaction, promoting the activation of immune cells against tumors, including NK cells, macrophages, and T cells. SIGLEC10 features a genuine immunoreceptor tyrosine-based inhibitory motif, dissimilar to other siglecs like SIGLEC15. In preclinical research, ONC-841 has demonstrated increased phagocytosis of cancer cells, enhanced functionality of tumor-infiltrating T cells and innate cells, and improved antibody-dependent CD16a signaling—a proxy for antibody-dependent cell-mediated cytotoxicity (ADCC).
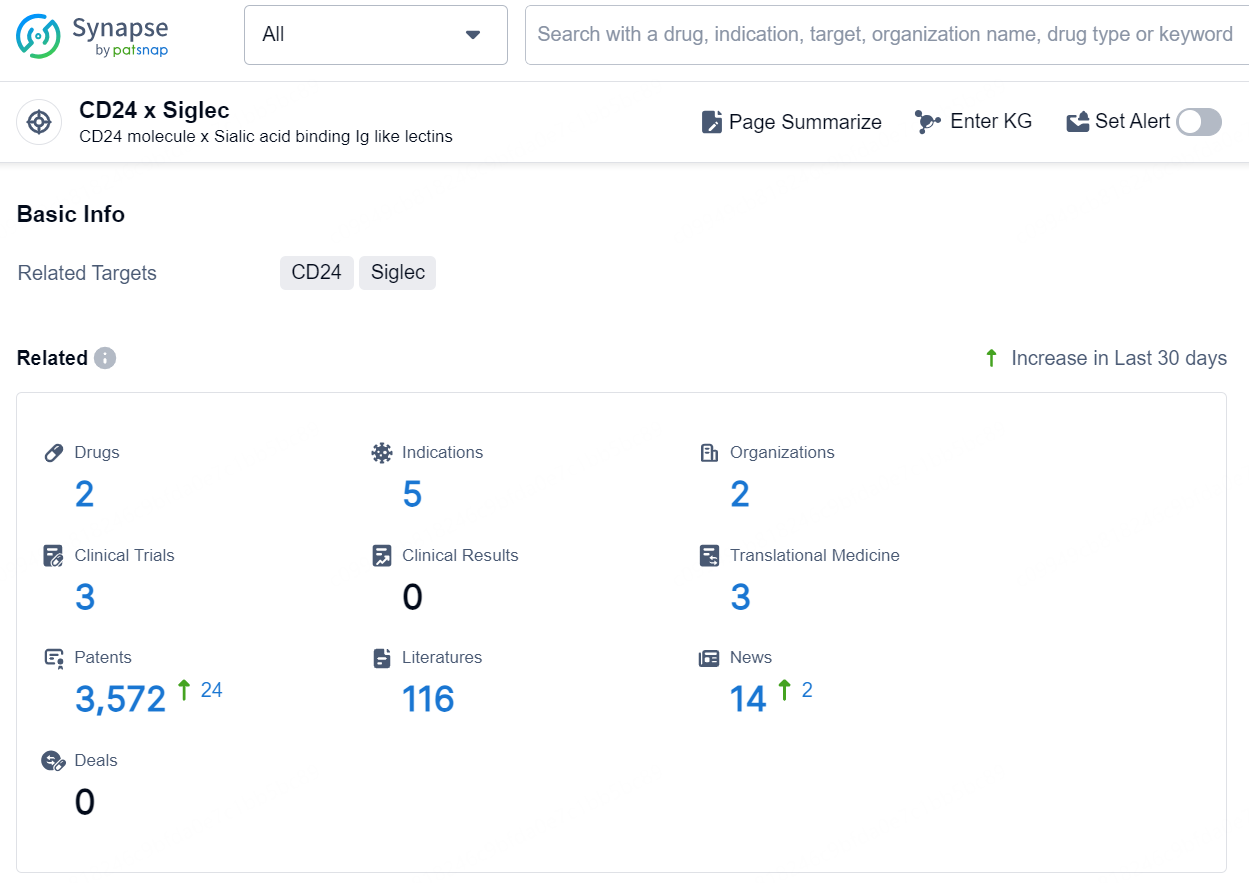
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 5, 2024, there are 2 investigational drugs for the CD24 x Siglec targets, including 5 indications, 2 R&D institutions involved, with related clinical trials reaching 3, and as many as 3572 patents.
The drug ONC-841 is a monoclonal antibody that targets CD24 x Siglec and is intended for use in the treatment of neoplasms, specifically advanced malignant solid neoplasms. The drug is currently in Phase 1 of development and is being developed by OncoC4, Inc. As a monoclonal antibody, ONC-841 is designed to target specific proteins on the surface of cancer cells, in this case, CD24 x Siglec. This targeted approach allows for more precise and effective treatment of the specific type of cancer for which it is intended. Neoplasms are abnormal growths or tumors, with malignant solid neoplasms being cancerous masses of tissue that do not contain any liquid or cystic areas. Thus, ONC-841 is being developed to address advanced stage cancer that has spread to solid tissues.