Orgenesis Announces Positive Results from ORG-101 CAR-T Study in CD19+ Acute Lymphoblastic Leukemia
Orgenesis Inc. (NASDAQ: ORGS) ("Orgenesis" or the "Company"), a global biotechnology enterprise dedicated to advancing the potential of cell and gene therapies (CGT) to enhance healthcare accessibility and outcomes, revealed encouraging results from a real-world clinical trial of its CD19 CAR-T therapy, ORG-101, in patients suffering from CD19+ Acute Lymphoblastic Leukemia (B-cell ALL). Harley Street Healthcare Group plans to establish a Global Cancer Initiative in collaboration with Orgenesis, aimed at democratizing Advanced Therapies and fostering further clinical advancements.
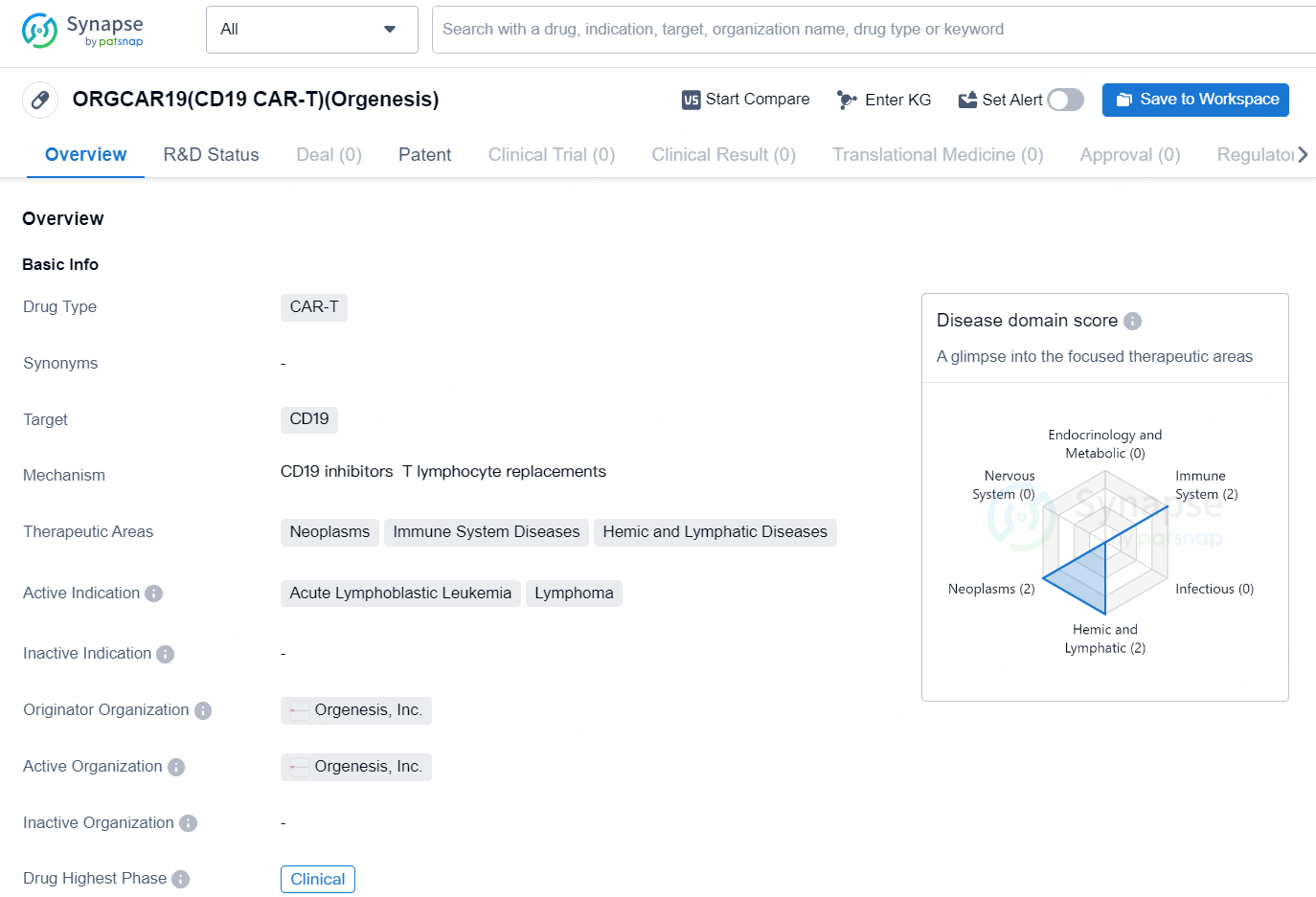
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The recent efficacy and safety data for the study reveal an impressive complete response (CR) rate of 82% in adult subjects and 93% in pediatric subjects. Additionally, the occurrence of severe Cytokine Release Syndrome (CRS)—a significant safety concern in CAR-T therapies—was found to be minimal, with rates of just 2% in adults and 6% in children, which is lower compared to existing treatments. This indicates a strong safety profile for ORG-101.
CRS is a systemic response caused by an excessive immune reaction to immunotherapy and poses a significant safety challenge in traditional CAR-T treatments. The findings, involving 233 patients from a real-world study at a prominent hematology center in China, support this safety profile. Further specifics are available in the company’s scientific presentation to be included as an exhibit in a Form 8-K filing.
Orgenesis is using its newly acquired GMP-Validated Platform to deploy the CAR-T therapy, adapting it to a decentralized manufacturing model. This model is intended to speed up capacity establishment, improve production efficiency, and lower treatment costs. The production data and cost analysis reinforce that Orgenesis’ decentralized approach offers a cost-effective alternative for accessing Advanced Therapies.
ORG-101 employs a third-generation lentiviral vector featuring a proprietary CAR construct, along with streamlined, decentralized onsite manufacturing and testing. This strategy presents a substantially more affordable option compared to traditional CAR-T therapies produced at centralized facilities.
The company has engaged in discussions with the FDA in the U.S., the Israeli Ministry of Health, and the Paul-Ehrlich-Institute in Germany regarding ORG-101. It is now gearing up to launch its own Phase 1/2 multicenter clinical trial, beginning at the General University Hospital of Patras in Greece and supported by its Enterprise Greece Grant, with plans to expand to additional sites within the Orgenesis network of hospital partnerships.
Vered Caplan, CEO of Orgenesis, stated, “We believe that these clinical results, combined with our GMP-Validated Platform, represent a significant advancement in our strategy to integrate our capabilities in decentralized cell therapy production with our regional partnerships. The product has not only demonstrated initial signs of positive clinical outcomes, but our production data also confirm that Orgenesis’ cost-effective decentralized cell processing can potentially enhance access to this treatment and reduce costs. We are committed to delivering this and other potentially life-saving treatments to patients globally.”
Sanjeev Kumar, Chief Visionary Officer at Harley Street Healthcare Group, outlined the objectives for the Global Cancer Initiative: “Cancer is increasingly affecting younger demographics. Our Global Cancer Initiative aims to gather contributions from various committed partners to make cancer therapies more affordable globally, focusing on democratizing Advanced Therapies. This initiative will strategically support our biobanking partnership and Orgenesis’ decentralized solutions to reduce costs and increase access to these therapies.”
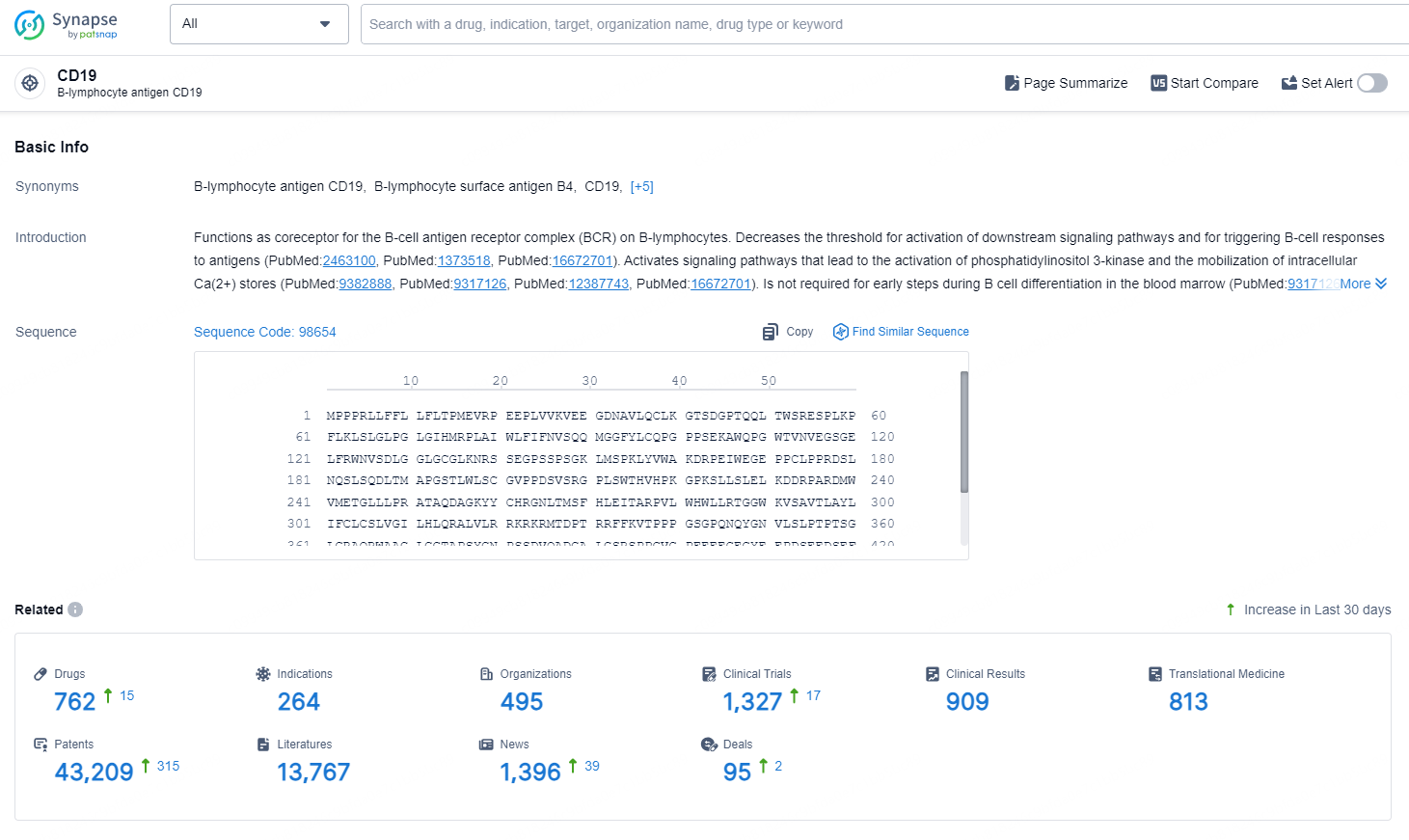
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of September 3, 2024, there are 762 investigational drugs for the CD19 target, including 264 indications, 495 R&D institutions involved, with related clinical trial reaching 1327, and as many as 43209 patents.
The drug ORGCAR19 (CD19 CAR-T) is a Chimeric Antigen Receptor T-cell (CAR-T) therapy that targets the CD19 antigen. It is being developed by Orgenesis, Inc. and is intended for the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases. The active indications for ORGCAR19 include acute lymphoblastic leukemia and lymphoma. CAR-T therapy involves modifying a patient's T cells to express chimeric antigen receptors that specifically target cancer cells. CD19 is a protein found on the surface of certain types of B-cell malignancies, making it an ideal target for CAR-T therapy in the treatment of hematologic cancers.