Patritumab Deruxtecan Improves Progression-Free Survival Over Doublet Chemotherapy in Advanced EGFR-Mutated NSCLC
The HERTHENA-Lung02 phase 3 study assessing patritumab deruxtecan in individuals with locally advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC) who previously underwent EGFR tyrosine kinase inhibitor (TKI) therapy reached its primary goal of progression-free survival (PFS), showing a statistically significant enhancement compared to platinum plus pemetrexed induction chemotherapy followed by pemetrexed maintenance chemotherapy. The data for overall survival (OS) were still immature at the analysis time, and the study will proceed to further evaluate OS as a secondary endpoint.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
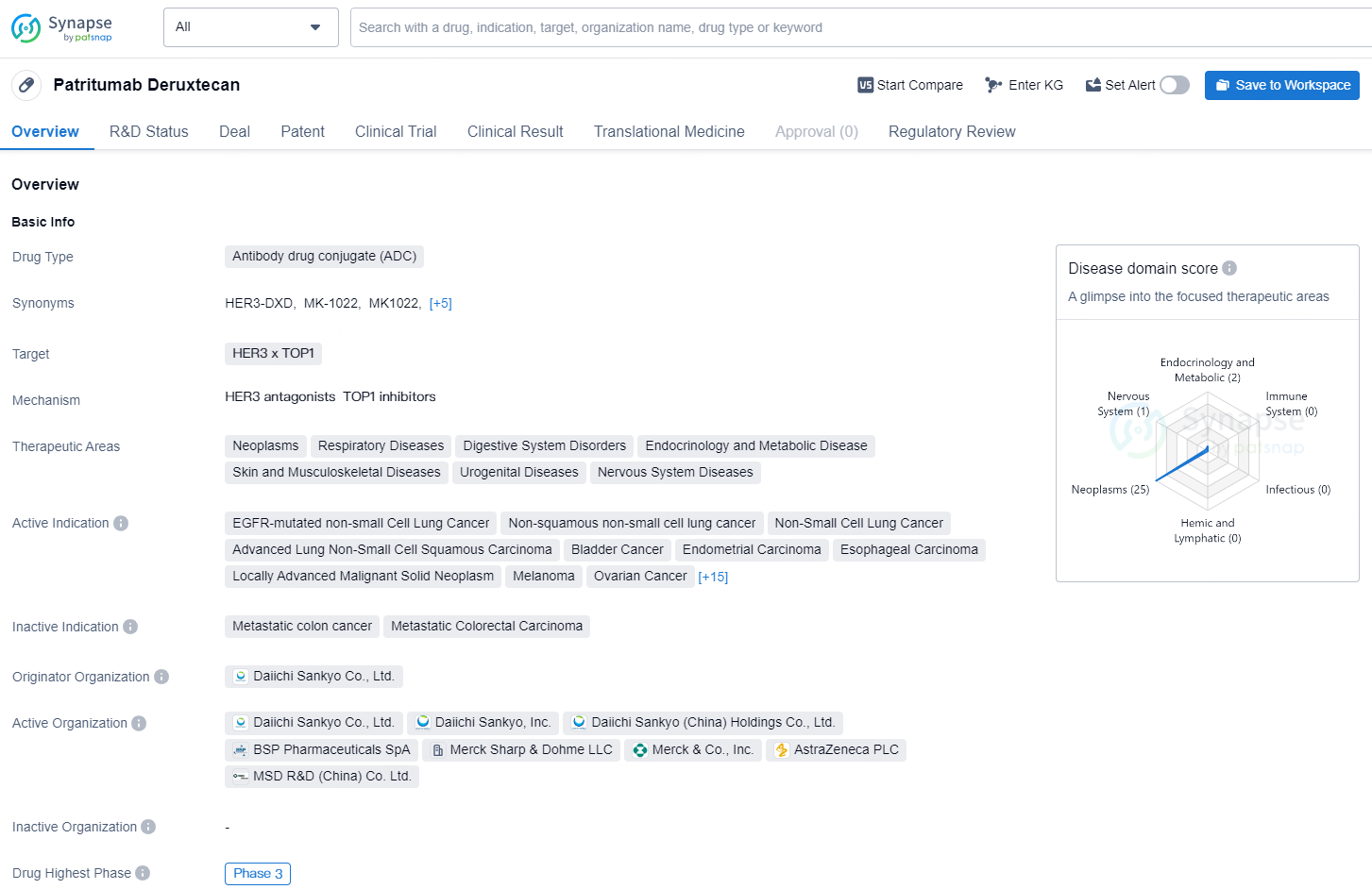
Patritumab deruxtecan is an engineered, first-in-class HER3 targeting DXd antibody drug conjugate (ADC) developed by Daiichi Sankyo (TSE: 4568) and co-developed with Merck (NYSE: MRK), known as MSD outside the US and Canada.
Globally, non-small cell lung cancer (NSCLC) makes up about 85% of all lung cancer cases, with up to 70% being diagnosed at an advanced stage. EGFR-activating mutations are present in 14% to 38% of NSCLC tumors worldwide. After initial treatment for metastatic EGFR-mutated NSCLC with an EGFR TKI, many patients face disease progression, and options in the second-line treatment setting are limited, underscoring the need for novel therapies to enhance patient outcomes.
Results from the HERTHENA-Lung02 study will be disseminated at an upcoming medical conference and provided to global regulatory bodies.
“The findings from HERTHENA-Lung02 suggest that patritumab deruxtecan has the potential to be a vital treatment option for certain EGFR-mutated non-small cell lung cancer patients who have previously undergone tyrosine kinase inhibitor treatment,” stated Ken Takeshita, MD, Global Head of R&D, Daiichi Sankyo. “We intend to discuss these results with regulatory authorities to determine subsequent steps.”
“We are motivated by these results showing a statistically significant improvement in progression-free survival compared to platinum plus pemetrexed induction chemotherapy followed by pemetrexed maintenance chemotherapy in patients with locally advanced or metastatic EGFR-mutated non-small cell lung cancer previously treated with a tyrosine kinase inhibitor,” said Marjorie Green, MD, Senior Vice President and Head of Oncology, Global Clinical Development, Merck Research Laboratories. “Together with Daiichi Sankyo, we are committed to addressing the high unmet need for patients with previously treated EGFR-mutated non-small cell lung cancer.”
The safety profile observed in HERTHENA-Lung02 was consistent with prior lung cancer clinical trials for patritumab deruxtecan, with no new safety signals detected. Most interstitial lung disease (ILD) events were low grade (grade 1 and 2). There were two observed cases of grade 5 ILD events.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
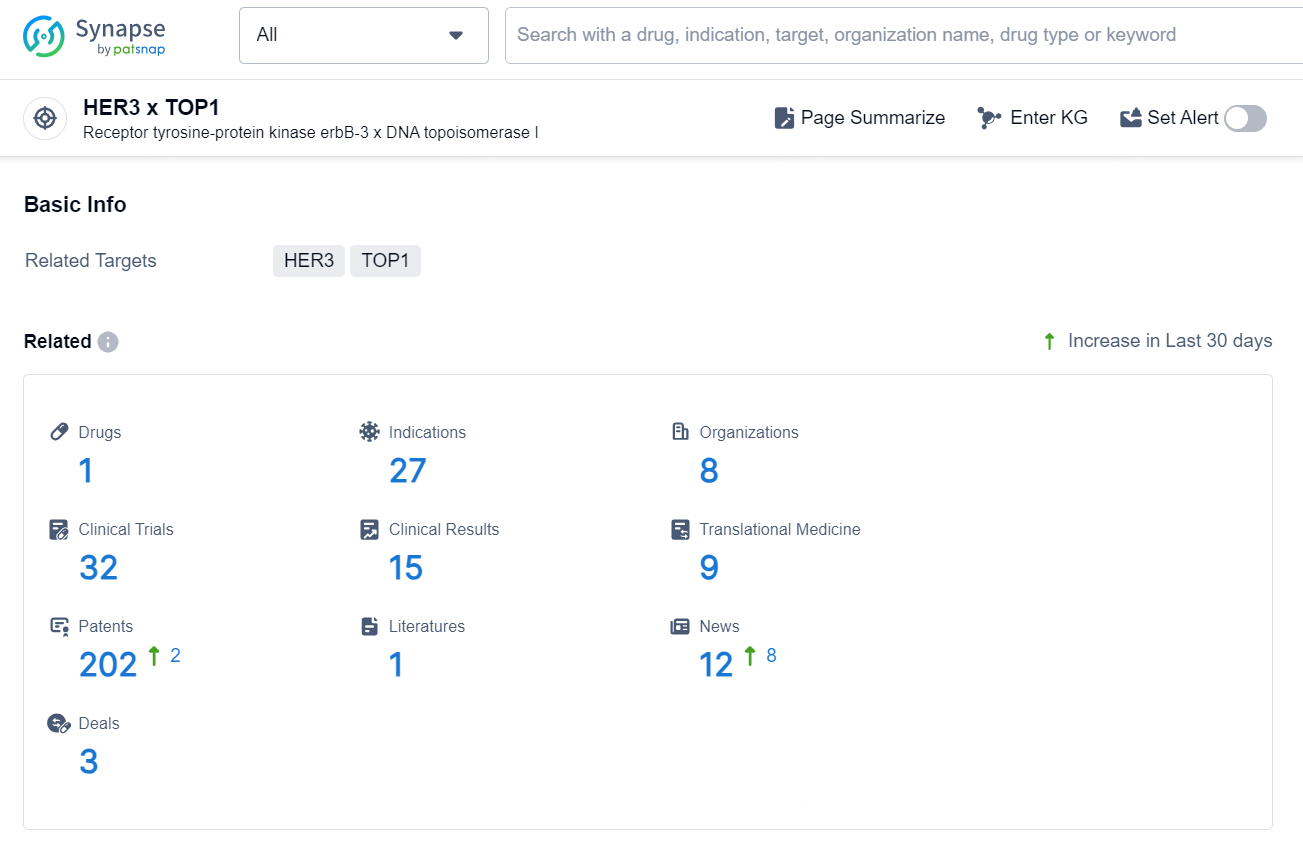
According to the data provided by the Synapse Database, As of September 18, 2024, there are 1 investigational drug for the HER3 and TOP1 target, including 27 indications, 8 R&D institutions involved, with related clinical trials reaching 32, and as many as 202 patents.
Patritumab Deruxtecan is an Antibody Drug Conjugate (ADC) developed by Daiichi Sankyo Co., Ltd., targeting HER3 and TOP1. Its primary indication is for the treatment of various neoplasms, respiratory diseases, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, urogenital diseases, and nervous system diseases. The drug is currently in Phase 3 of development globally and in China.