Pharmaceutical Insights: Disitamab Vedotin's R&D Progress and its Mechanism of Action on Drug Target
Disitamab Vedotin's R&D Progress
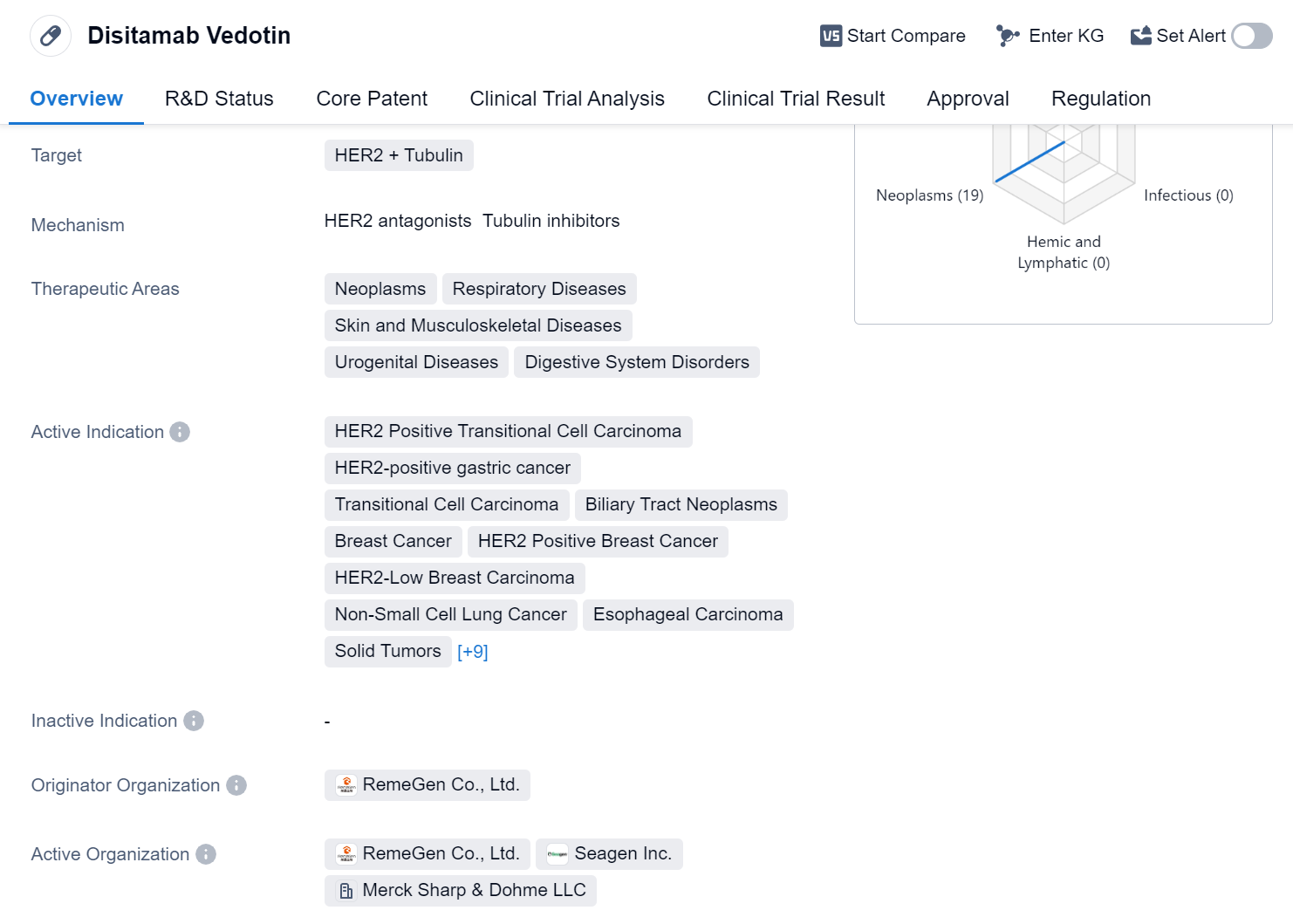
Disitamab Vedotin is a monoclonal antibody and antibody drug conjugate (ADC) that targets HER2 and Tubulin. It is primarily used in the treatment of various neoplasms, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, and digestive system disorders. The drug has shown efficacy in treating HER2 Positive Transitional Cell Carcinoma, HER2-positive gastric cancer, Transitional Cell Carcinoma, Biliary Tract Neoplasms, Breast Cancer, HER2 Positive Breast Cancer, HER2-Low Breast Carcinoma, Non-Small Cell Lung Cancer, Esophageal Carcinoma, Solid Tumors, Uterine Cervical Cancer, HR-positive/HER2-low Breast Carcinoma, HR-negative/HER2-low Breast Carcinoma, Non-Muscle Invasive Bladder Neoplasms, HER2 positive Invasive Bladder Carcinoma, Melanoma, HER2 mutant non-small cell lung cancer, HER2 Positive Solid Tumors, and Stomach Cancer.
Disitamab Vedotin was developed by RemeGen Co., Ltd., and it has received approval for use in global market. The drug obtained its first approval in China in June 2021. It has undergone rigorous regulatory processes, including priority review, conditional marketing approval, fast track designation, orphan drug status, and breakthrough therapy designation.
The approval of Disitamab Vedotin marks a significant milestone in the pharmaceutical industry, as it offers a promising treatment option for patients with various types of cancers and other diseases. The drug's mechanism of action, targeting HER2 and Tubulin, makes it a valuable addition to the existing treatment options available.
The wide range of therapeutic areas covered by Disitamab Vedotin highlights its potential to address multiple unmet medical needs. Its approval for different indications, including breast cancer, lung cancer, bladder neoplasms, and stomach cancer, demonstrates its versatility in treating various types of tumors. This versatility is further supported by its ability to target HER2 mutations and HER2-low breast carcinoma, which are challenging to treat with conventional therapies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Disitamab Vedotin: HER2 antagonists & Tubulin inhibitors
HER2 antagonists are a type of medication that specifically target and block the activity of the HER2 protein. HER2, also known as human epidermal growth factor receptor 2, is a protein that plays a role in cell growth and division. In some cases, HER2 can be overexpressed in certain types of cancer cells, such as breast cancer cells, leading to uncontrolled cell growth and tumor formation.
HER2 antagonists work by binding to the HER2 protein and preventing it from sending signals that promote cell growth and division. By inhibiting the activity of HER2, these medications can help slow down or stop the growth of cancer cells that rely on HER2 signaling for their survival.
Tubulin inhibitors, on the other hand, are a class of drugs that interfere with the function of tubulin, a protein involved in cell division. Tubulin is responsible for forming the structural framework, called microtubules, that help separate chromosomes during cell division.
Tubulin inhibitors can disrupt the formation of microtubules, preventing proper chromosome separation and ultimately leading to cell death. These drugs are commonly used in cancer treatment because rapidly dividing cancer cells are particularly sensitive to the disruption of cell division.
In summary, HER2 antagonists specifically target the HER2 protein to inhibit its activity and slow down cancer cell growth, while tubulin inhibitors interfere with the function of tubulin to disrupt cell division and induce cell death. Both types of medications are important in the field of biomedicine for the treatment of certain types of cancer.
Drug Target R&D Trends for Disitamab Vedotin
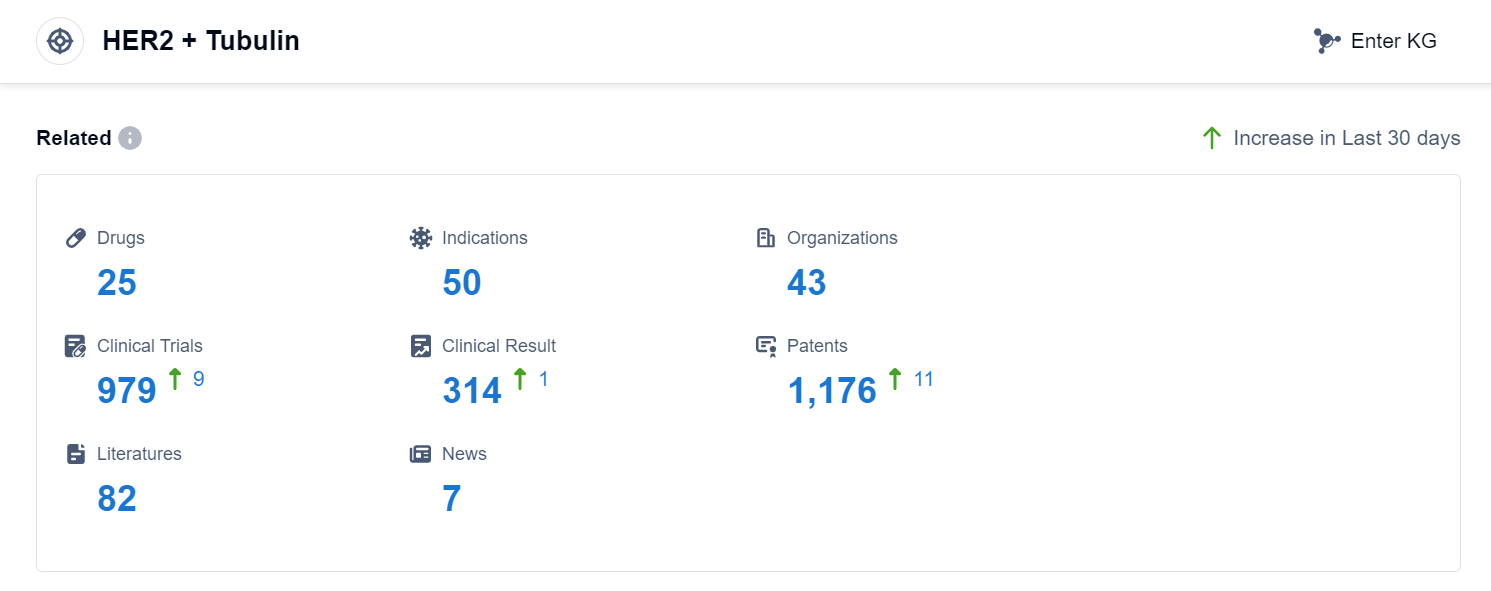
According to Patsnap Synapse, as of 3 Sep 2023, there are a total of 25 HER2 + Tubulin drugs worldwide, from 43 organizations, covering 50 indications, and conducting 979 clinical trials.
Overall, the target HER2 + Tubulin shows a promising competitive landscape with active R&D progress and multiple drugs approved for relevant indications. Further analysis of specific drugs, their mechanisms of action, and clinical trial results would provide more insights into the future development of this target.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Disitamab Vedotin's approval and its unique characteristics as a monoclonal antibody and ADC targeting HER2 and Tubulin make it a promising drug in the field of biomedicine. Its potential to improve patient outcomes in multiple therapeutic areas and its regulatory designations further enhance its value in the pharmaceutical industry.