Phase Ib study of AskBio's AB-1005 genetic treatment for Parkinson’s disease achieves main objective
Bayer AG, in conjunction with its wholly owned but autonomously managed subsidiary Asklepios BioPharmaceutical, Inc., specializing in gene therapy, has confirmed the finalization of data assembly over a period of 18 months for the initial Phase Ib human trial concerning AB-1005 (AAV2-GDNF). This pioneering genetic treatment is being evaluated for its potential to alleviate symptoms in individuals afflicted with Parkinson’s disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The research successfully achieved its main goal, which involved assessing the safe application of a single bilateral administration of AB-1005 directly into the putamen. A total of eleven participants were segmented into two distinct groups, one representing individuals with Mild stage Parkinson's disease (PD) and the other with Moderate stage PD. Classification into these groups was determined by the duration since a PD diagnosis and the intensity of PD manifestations observed during the initial assessment.
The neurosurgical administration of AB-1005 was positively received by the patients, attaining an average target coverage of the putamen of 63% with a standard deviation of 2%, surpassing the anticipated aim of over 50% coverage using AB-1005. To date, none of the serious adverse events reported have been linked to AB-1005, with a continued clinical monitoring period extending up to five years after the treatment is in progress.
"These preliminary results are promising, indicating that AB-1005 is acceptable in patients diagnosed with mild to moderate PD," commented Krystof Bankiewicz, MD, PhD, the lead for Parkinson’s and MSA research at AskBio. "While there's still a considerable amount to discover about this gene therapy that's still in the early stages of investigation, these initial outcomes are set to shape our ongoing research in this domain. They hold the potential to be instrumental in the future clinical development of AB-1005 as a treatment for PD."
"Offering new treatment avenues for individuals struggling with PD is paramount," expressed Christian Rommel, PhD, a key figure on Bayer’s Pharmaceuticals Division's Executive Committee, taking charge of Research and Development. "This favorable result from the Phase Ib trial of AB-1005 marks a pivotal progression in our dedication to providing innovative treatments in domains where there's the capacity to effect significant change."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
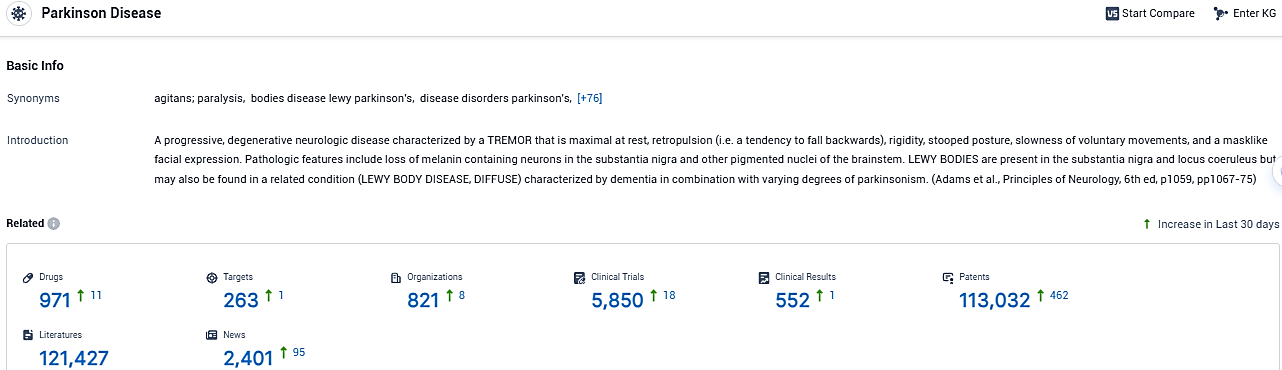
According to the data provided by the Synapse Database, As of January 9, 2024, there are 971 investigational drugs for Parkinson’s disease, including 263 targets, 821 R&D institutions involved, with related clinical trials reaching 5850, and as many as 113032 patents.
AB-1005 is an investigational gene therapy based on adeno-associated viral vector serotype 2 containing the human glial cell line-derived neurotrophic factor transgene, which allows for stable and continuous expression of GDNF in localized regions of the brain after direct neurosurgical injection with MRI-monitored convection enhanced delivery. GDNF has long been evaluated as a potential treatment for diseases, such as Parkinson’s, marked by progressive degeneration of midbrain dopaminergic neurons.