Promising Phase 2 Results for Ionis's ION224 in NASH/MASH Treatment
Ionis Pharmaceuticals, Inc. has released encouraging outcomes from its Phase 2 clinical trial that evaluated ION224, a research-stage DGAT2 antisense inhibitor aimed at addressing metabolic dysfunction-associated steatohepatitis, which is also known as nonalcoholic steatohepatitis. The research hit its main goal with both administered quantities – 120 mg and 90 mg – demonstrating significant histologic enhancements in liver condition. Moreover, the trial successfully reached a crucial secondary goal, which was the dissolution of MASH.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The initial findings from the second phase of clinical testing for ION224 indicate a positive correlation between decreased liver fat levels following DGAT2 suppression and improved histological markers for MASH, as noted by Rohit Loomba, MD, MHSc. Dr. Loomba, a medicine professor and head of the gastroenterology and hepatology division at UC San Diego, expressed optimism for ION224's potential as a precise therapeutic option for MASH. It may offer a synergistic effect alongside other candidates being tested for this condition. Dr. Loomba is keen on the further evaluation of this promising medicine.
MASH represents a severe variant of metabolic dysfunction-associated fatty liver disease, elevating risks for liver fibrosis, cirrhosis, and mortality due to liver complications. As a cutting-edge LIgand-Conjugated Antisense treatment, ION224 aims to lessen diacylglycerol acyltransferase 2 production in MASH patients.
Sanjay Bhanot, MD, PhD, vice president and head medical officer at Ionis, highlighted the uniqueness of DGAT2 inhibition as a therapeutic strategy in the battle against progressive conditions like MASH, which is in dire need of efficacious treatments. Dr. Bhanot expressed eagerness to unveil comprehensive results from the research at a forthcoming scientific meeting and to deliberate future directions to enhance this promising treatment for those affected.
Safety profiles from the research indicate that ION224 has been received favorably by subjects with MASH. No significant adverse effects on the liver or kidneys, nor gastrointestinal issues, were reported by participants receiving ION224. Furthermore, this arm experienced fewer instances of early study withdrawal in comparison to the placebo group. Additionally, there were zero deaths or serious adverse events associated with the treatment during the study.
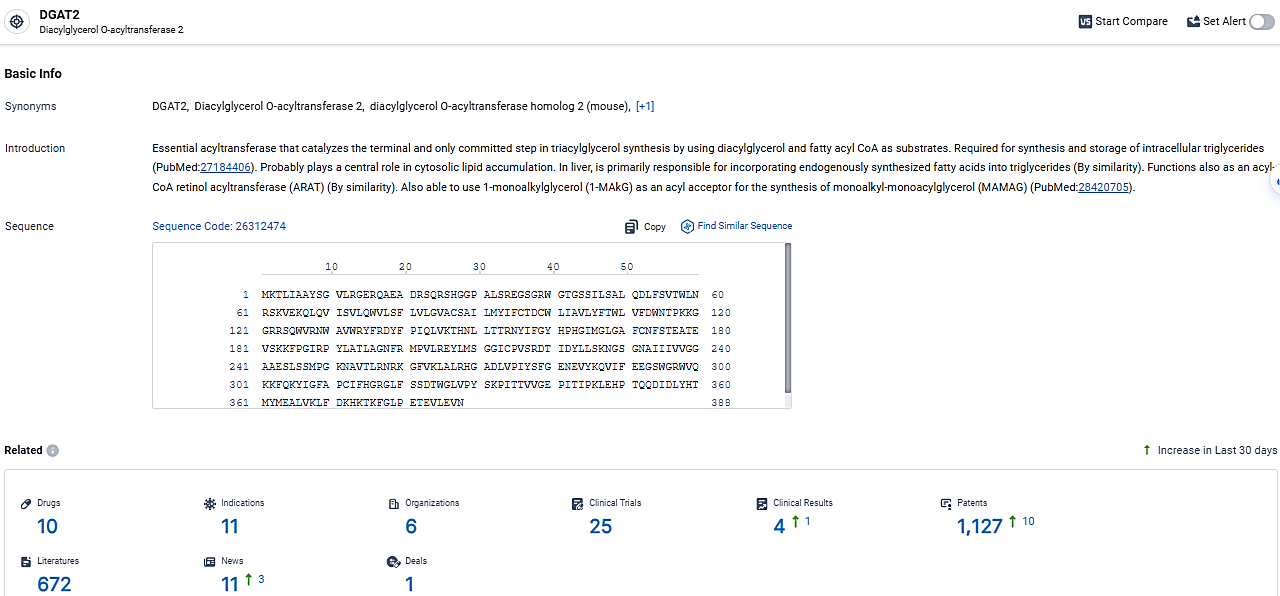
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 17, 2024, there are 10 investigational drugs for the DGAT2 target, including 11 indications, 6 R&D institutions involved, with related clinical trials reaching 25, and as many as 1127 patents.
ION224 is an investigational LIgand-Conjugated Antisense medicine designed to reduce the production of diacylglycerol acyltransferase 2 to treat patients with MASH. DGAT2 is an enzyme that catalyzes the final step in triglyceride synthesis in the liver. Reducing the production of DGAT2 should therefore decrease triglyceride synthesis in the liver. ION224 offers a unique approach, which is potentially complementary to other therapies currently in clinical development.