REGENXBIO Reports Positive Results for RGX-121, Showing Lasting Systemic Impact
REGENXBIO Inc. (Nasdaq: RGNX) revealed encouraging findings from the Phase I/II/III CAMPSIITE® study of RGX-121, aimed at treating Mucopolysaccharidosis Type II (MPS II), commonly referred to as Hunter syndrome. These outcomes were shared at the 2024 Annual Symposium of the Society for the Study of Inborn Errors of Metabolism (SSIEM).
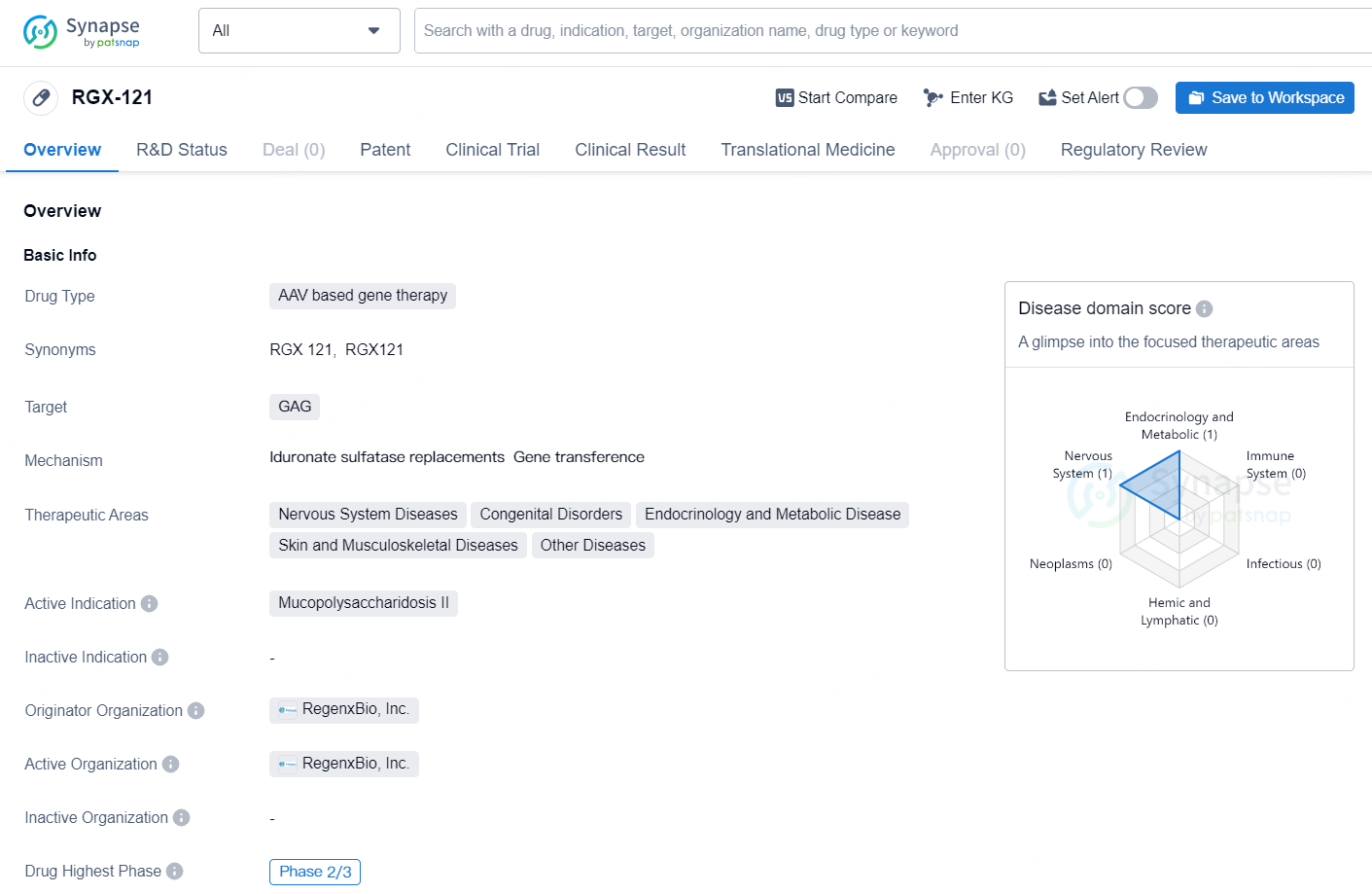
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The extensive evidence gathered from the CAMPSIITE trial continues to endorse RGX-121 as a promising pioneer gene therapy and a one-time treatment for MPS II. Within the United States, RGX-121 is poised to become the inaugural treatment targeting the neurocognitive decline seen in MPS II, and it holds potential to be the preferred treatment for patients with the neuronopathic form of the disease.
"As we swiftly approach the BLA submission for RGX-121, we are thrilled with the data unveiled at SSIEM, showcasing promising signs of systemic activity and enduring reductions of CSF D2S6," said Steve Pakola, M.D., Chief Medical Officer at REGENXBIO. "The results further indicate that by reinstating the missing gene in boys affected by Hunter syndrome, RGX-121 alters the disease's trajectory and holds the potential to markedly enhance both critical brain functions and the systemic symptoms of this debilitating condition."
Data Overview
Recent long-term data from the Phase I/II/III CAMPSIITE® trial show that patients administered RGX-121 at the critical dose level achieved a median 85% reduction in cerebrospinal fluid (CSF) levels of heparan sulfate (HS) D2S6, an essential biomarker of brain disease activity, nearing normal levels and maintained for up to two years. Preliminary results released earlier this year from the CAMPSIITE trial indicated that the critical phase of the trial reached its primary endpoint with statistical significance. These pivotal findings were in line with those from the dose-finding phase of CAMPSIITE, where most patients exceeded expectations in neurodevelopmental performance when compared to natural history data over a span of up to four years.
During the dose-finding phase of the trial, researchers opted to discontinue the standard intravenous enzyme replacement therapy (ERT) or to keep most patients ERT-naïve. At the critical dose level (dose level 3), 80% of patients remained ERT-free at the last evaluation point, over 18 months post-dosing. At dose level 2, 71% of patients were ERT-free at the last evaluation point, nearly three years post-dosing.
As of January 5, 2024, RGX-121 continues to be well-tolerated in 25 patients across all stages of the CAMPSIITE trial.
"A potential one-time therapy that allows these boys to surpass the natural history of their neurocognitive development and remain off enzyme replacement therapy for several years represents a significant option for patients and their families," said Roberto Giugliani, M.D., Ph.D., Professor, Department of Genetics, UFRGS, Medical Genetics Service, HCPA, Porto Alegre, Brazil. "I remain very optimistic about the data supporting RGX-121 and look forward to seeing this program progress toward potential approval for this patient community."
REGENXBIO is scheduled to begin a rolling Biologics License Application (BLA) submission via the accelerated approval pathway in Q3 2024, utilizing CSF D2S6 as a surrogate endpoint reasonably likely to predict clinical benefit. Approval of this BLA could lead to the granting of a Priority Review Voucher in 2025.
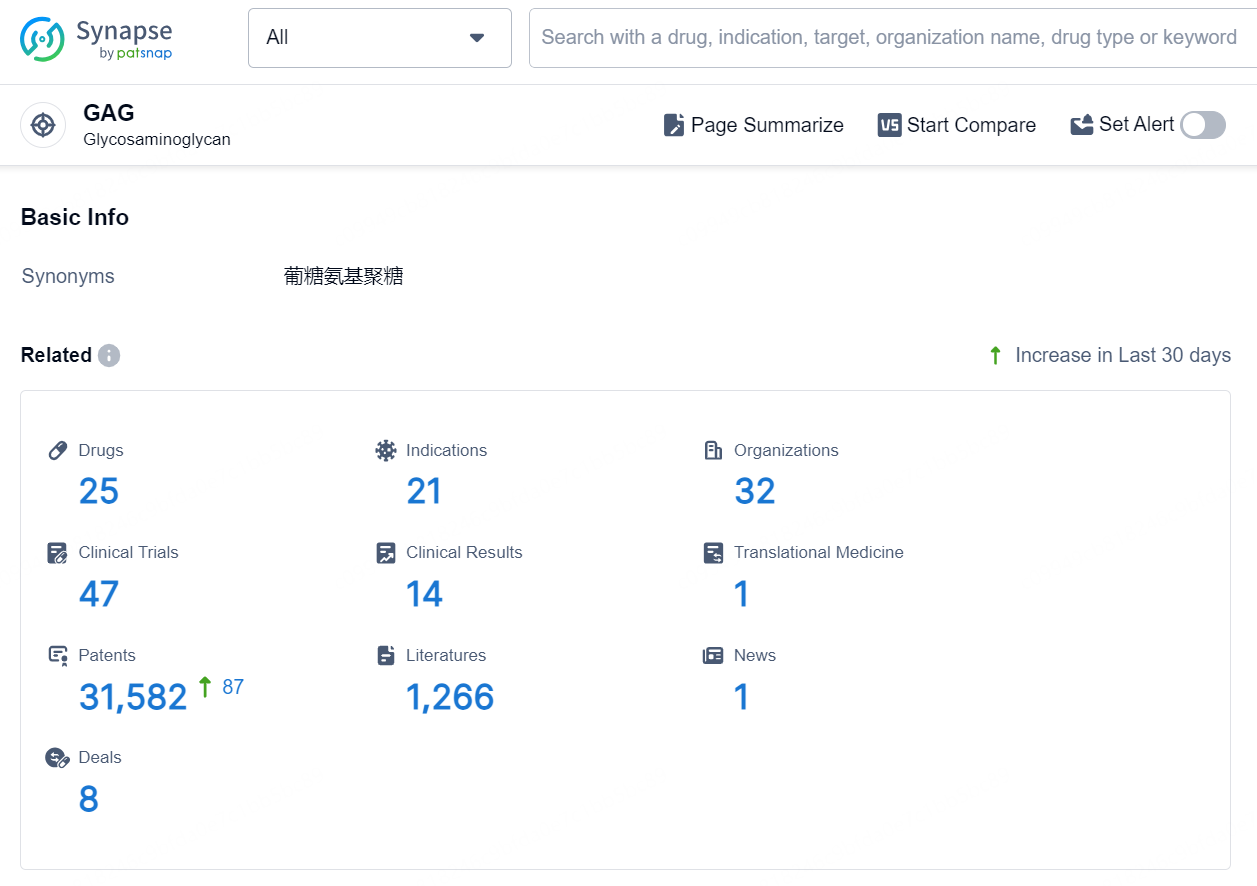
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 5, 2024, there are 25 investigational drugs for the GAG target, including 21 indications, 32 R&D institutions involved, with related clinical trials reaching 47, and as many as 31582 patents.
RGX-121 is an AAV based gene therapy drug developed by RegenxBio, Inc. The drug is designed to target the GAG and is intended for the treatment of Mucopolysaccharidosis II, also known as MPS II or Hunter syndrome. MPS II is a rare genetic disorder that primarily affects males and is characterized by the accumulation of glycosaminoglycans (GAG) in various tissues and organs, leading to a range of debilitating symptoms.