Revolutionizing Medicine: Advances in siRNA Drugs and Delivery Technologies
Small interfering RNA (siRNA) drugs, as a novel class of therapeutics based on the RNA interference (RNAi) mechanism, have achieved significant advancements in the biotechnology field in recent years. According to Maximize Market Research, the siRNA therapeutics market is projected to grow substantially from $12.7 billion in 2023 to $39.2 billion by 2029, with a remarkable compound annual growth rate (CAGR) of 25.3%. This growth is primarily driven by the widespread application of siRNA drugs in treating various diseases and the continuous advancements in delivery technologies. North America is currently the largest contributor to the siRNA drug market, particularly the United States, which had a market size of $902 million in 2023 and is expected to reach $5.221 billion by 2030, with a CAGR of 25.58%. The European market is also notable, with a size of $506 million in 2023, anticipated to grow to $4.541 billion by 2030, representing a CAGR of 34.51%.
Delivery Methods for siRNA Drugs
As an emerging therapeutic approach, siRNA drugs offer high specificity and efficacy but face numerous challenges in practical application, especially regarding the efficient and safe delivery to target cells. Currently, the primary delivery systems include lipid nanoparticles (LNPs) and N-acetylgalactosamine (GalNAc) conjugation technology. LNPs enable siRNA delivery to various tissues, while GalNAc conjugation facilitates liver-specific delivery. With the advancement of nanotechnology and biomaterials science, more efficient delivery systems, such as polymer-based nanoparticles, exosomes, and viral vectors, are expected to be developed. These innovative technologies will further enhance the delivery efficiency and safety of siRNA therapeutics.
Structure and Composition of Lipid Nanoparticles (LNPs)
LNPs are multi-component lipid formulations designed to encapsulate siRNA, protecting it from degradation in the bloodstream and facilitating its transmembrane delivery into target cells. This delivery system demonstrates exceptional performance in enhancing the stability and targeting ability of siRNA and has been applied in several siRNA therapeutics, such as Patisiran.
Ionizable lipids are among the most critical components of LNPs, exhibiting distinct charge characteristics under different pH conditions. Under acidic conditions (e.g., within endosomes), ionizable lipids carry a positive charge, promoting the formation of complexes with negatively charged siRNA. Under physiological pH, ionizable lipids are mostly neutral, helping to minimize immune responses and cytotoxicity. The molecular structure of ionizable lipids includes a charged head group, linker segments, and hydrophobic tails. The charge density and lipophilicity of the head group are crucial for LNP performance. For instance, the head groups of ALC-0315 and SM-102 contain terminal hydroxyl groups that reduce hydration effects, enhancing hydrogen bonding interactions with siRNA and improving transfection efficiency.
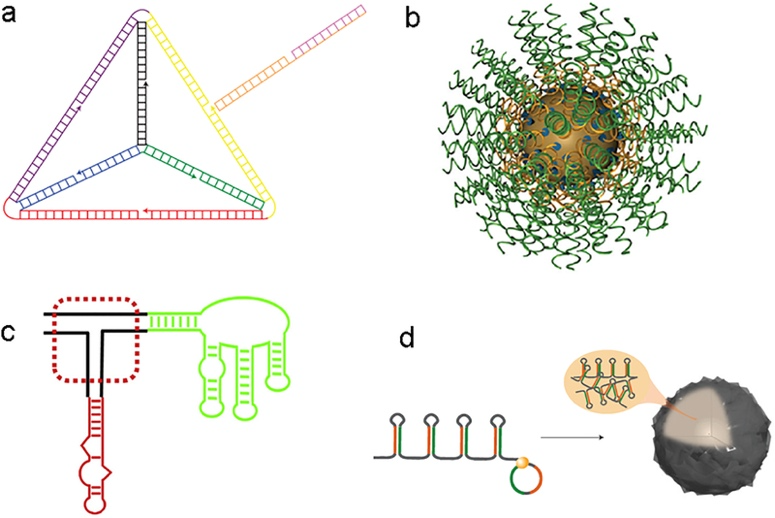
Cholesterol enhances the structural stability and rigidity of LNPs, helping to prevent siRNA leakage. The cholesterol content in LNPs typically ranges from 20% to 50%, and optimizing its proportion can improve LNP stability and delivery efficiency. Helper lipids, often neutral or cationic lipids, contribute to forming a stable lipid bilayer structure. Commonly used helper lipids include 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), which help maintain the structural integrity and stability of LNPs. Polyethylene glycol (PEG)-modified lipids increase the solubility and stability of LNPs, reducing recognition and clearance by the immune system and prolonging circulation time in the bloodstream. The hydrophilic shell provided by PEG-modified lipids forms a "stealth" barrier that prevents rapid clearance by the mononuclear phagocyte system (MPS), enhancing LNP bioavailability and delivery efficiency.
The mechanism of action of LNPs in the application of siRNA drugs
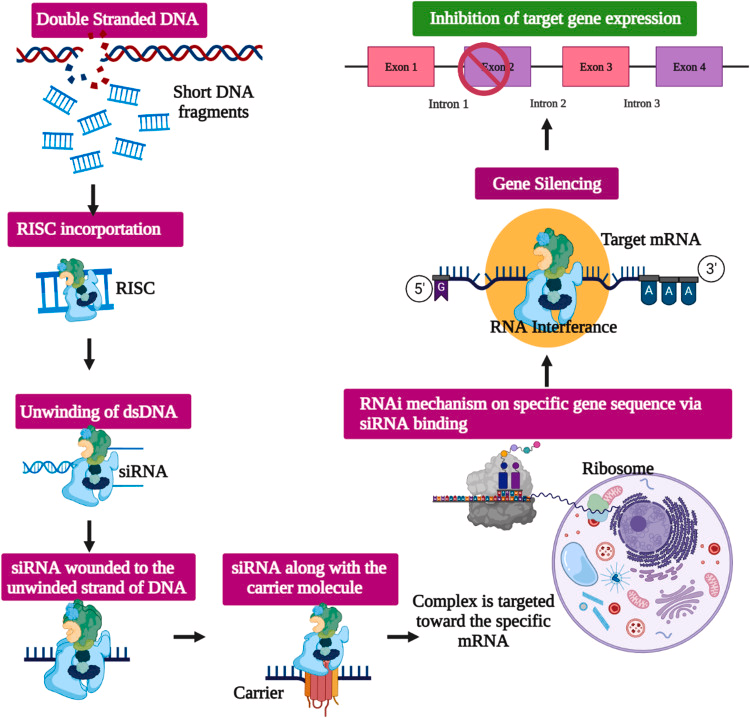
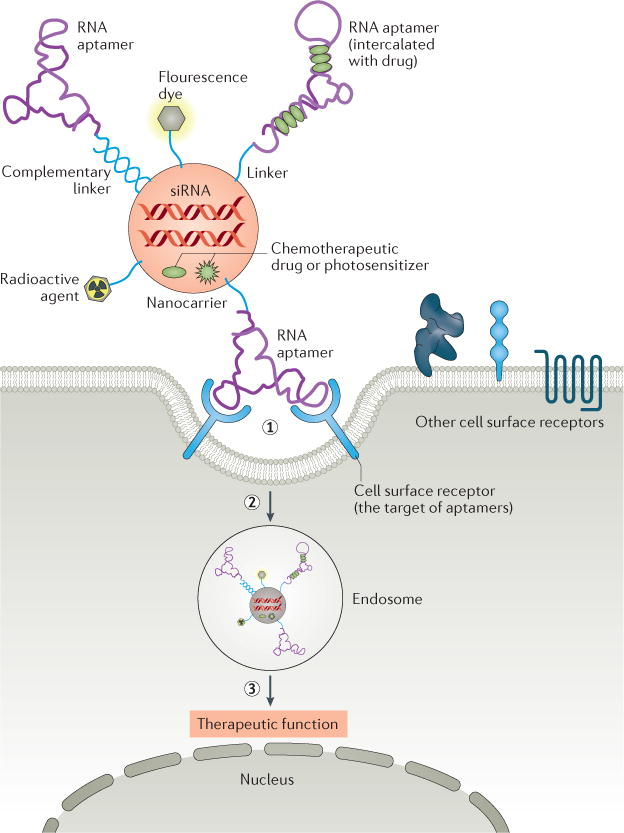
During LNP preparation, siRNA forms complexes with ionizable lipids via electrostatic interactions and is encapsulated within the lipid bilayer, effectively protecting the siRNA from degradation by nucleases in the plasma. PEG modification on the LNP surface extends circulation time and reduces immune recognition. LNPs deliver siRNA to specific cells or tissues via passive or active targeting mechanisms. Active targeting typically involves ligands such as GalNAc that bind specifically to receptors on target cell surfaces. Once LNPs are internalized by endocytosis, the acidic environment within endosomes activates ionizable lipids to disrupt the endosomal membrane, releasing siRNA into the cytoplasm. The siRNA then assembles with proteins like Argonaute-2 to form the RNA-induced silencing complex (RISC), which silences specific genes by cleaving or inhibiting the translation of target mRNA, achieving therapeutic effects.
Despite significant advancements in LNP-mediated siRNA delivery, challenges remain, including improving delivery efficiency, reducing side effects, and achieving broader tissue targeting. Future research will focus on enhancing LNP stability and targeting specificity through the design and screening of new lipid components, such as ionizable lipids with higher pH sensitivity and lower toxicity, and biocompatible substitutes for helper lipids and cholesterol. Surface modifications with antibodies, peptides, or small molecule ligands could enable more precise cell or tissue-specific binding, minimizing off-target effects and improving safety and efficiency. The integration of diverse delivery technologies, such as microneedles or oral formulations, could broaden application scenarios, including inhalable or oral LNP systems for respiratory or gastrointestinal diseases. Additionally, advances in gene sequencing and bioinformatics could facilitate the design of personalized siRNA sequences and delivery strategies tailored to individual patient differences, driving the development of precision medicine.
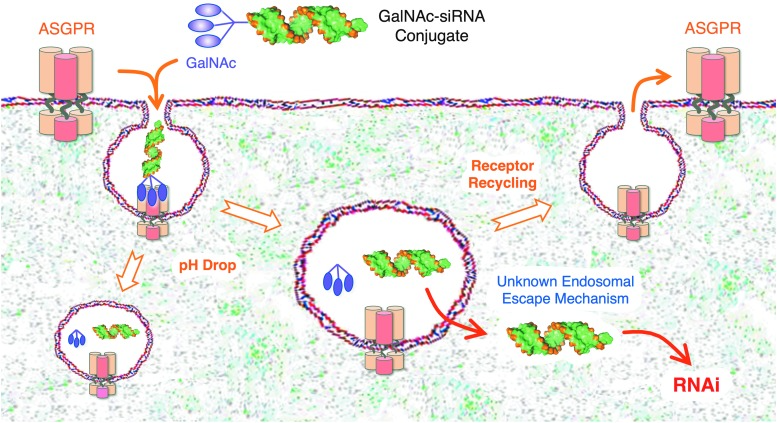
The Mechanism of GalNAc in siRNA Drug Delivery
GalNAc (N-acetylgalactosamine) is a naturally occurring sugar molecule with high affinity for the asialoglycoprotein receptor (ASGPR) on the surface of hepatocytes. ASGPR, which is highly expressed on liver cells and functions as an endocytic receptor, facilitates the transport of bound ligands into the cell. The strong binding affinity between GalNAc and ASGPR allows for specific targeting of hepatocytes, enabling efficient siRNA delivery. Although ASGPR is also expressed in certain other cell types, its expression level is significantly lower outside the liver, ensuring that GalNAc primarily targets hepatocytes and minimizes non-specific uptake by non-target cells.
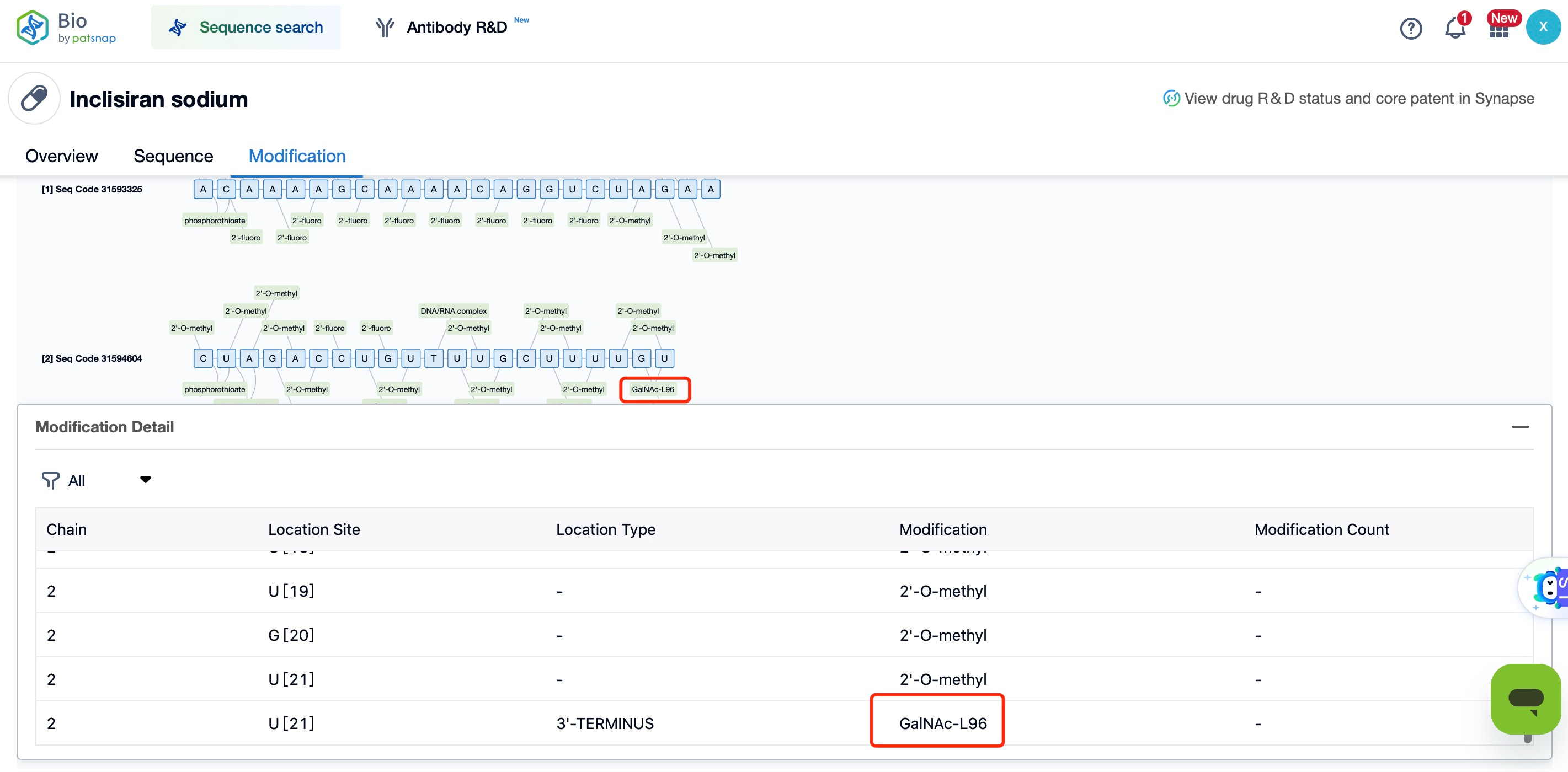
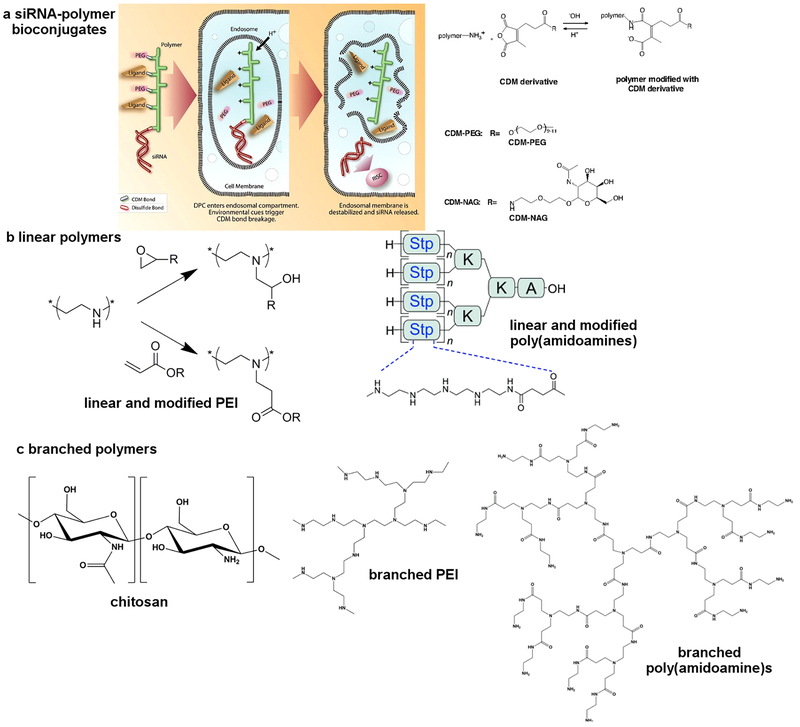
By chemical modification, GalNAc can be covalently attached to the 3' end of siRNA, forming a GalNAc-siRNA conjugate. This conjugate remains stable in circulation, resisting degradation, which ensures the siRNA reaches its target cells effectively. The covalent attachment process involves multiple steps, typically employing a linker that connects the GalNAc molecule to the siRNA's 3' end. The design of the linker is critical—it must ensure stable attachment while also allowing the siRNA to be released under intracellular conditions. Common linkers include disulfide bonds and ester bonds, which are designed to cleave in the cell’s reductive or acidic environments, thereby releasing the siRNA.
To enhance delivery efficiency, multiple GalNAc molecules are often attached to the 3' end of the sense strand of siRNA, creating a multivalent conjugate. Multivalent conjugates improve binding affinity to ASGPR and increase cellular uptake efficiency. Studies have shown that trivalent GalNAc conjugates offer the highest delivery efficiency and are widely used in practice. This multivalent design not only enhances siRNA delivery but also reduces the required dose, minimizing potential side effects.
When GalNAc-siRNA conjugates enter the bloodstream, the GalNAc moiety binds specifically to ASGPR receptors on the surface of hepatocytes. Through clathrin-mediated endocytosis, the conjugates are internalized into cells, forming endosomes. As the endosomal environment acidifies, the decreasing pH triggers the cleavage or hydrolysis of the linker, such as disulfide or ester bonds, under reductive or acidic conditions, releasing the siRNA. To enhance siRNA escape from endosomes into the cytoplasm, researchers have developed various strategies, including incorporating cationic lipids or peptides to destabilize the endosomal membrane and optimizing the physicochemical properties of the conjugate, such as its size and shape. These methods collectively improve the endosomal escape efficiency, increase the bioavailability of siRNA in the cytoplasm, and significantly enhance the overall delivery efficiency of GalNAc-siRNA conjugates.
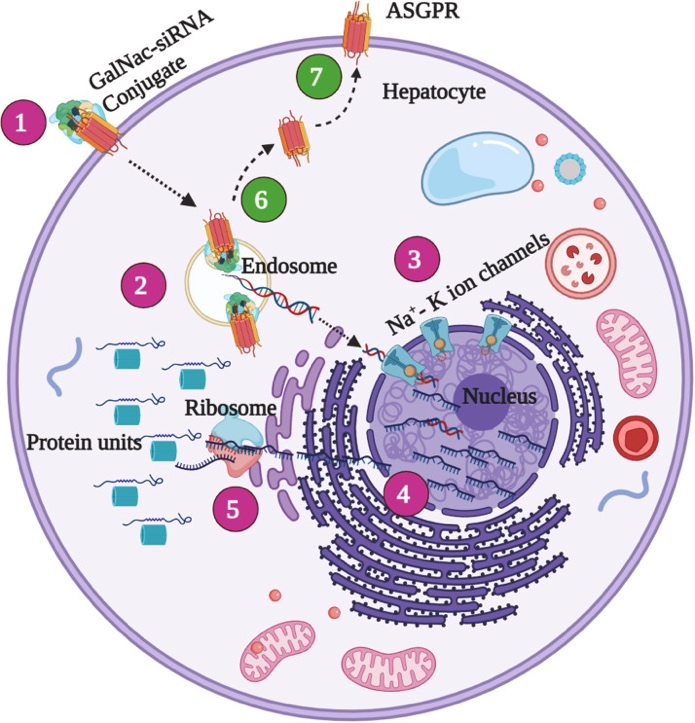
GalNAc conjugation technology achieves efficient siRNA delivery through specific hepatocyte targeting, effective cellular uptake and endosomal escape, and precise gene-silencing mechanisms. This technology has been applied to multiple drugs, demonstrating significant potential. Future research will further optimize GalNAc conjugate design to enhance delivery efficiency, reduce side effects, and expand its application to more disease areas.
Application of Polymer Nanoparticles (PNPs) in siRNA Drug Delivery
PNPs are an efficient siRNA delivery system that forms stable nano-complexes by binding with siRNA, protecting it from degradation. They enable precise delivery to specific cells or tissues via mechanisms including blood circulation, targeted delivery, cellular uptake, and gene silencing. Common polymers include cationic polymers (e.g., PEI), biodegradable polymers (e.g., PLGA), and dendrimers (e.g., PAMAM). Fabrication methods include self-assembly, emulsion-solvent evaporation, and microfluidics. PNPs have shown great potential in treating cancer, cardiovascular diseases, and viral infections. However, challenges remain, including delivery efficiency, cytotoxicity, and immune responses. Future research will focus on optimizing polymer structures, surface modifications, and multimodal delivery techniques to improve their safety and efficacy.
Conclusion
siRNA-based drugs leverage RNA interference mechanisms, offering high specificity and efficacy in treating various diseases. However, achieving efficient and safe siRNA delivery to target cells remains a major challenge. While delivery systems such as LNPs, GalNAc conjugates, and PNPs have significantly improved siRNA stability and targeting, continuous optimization is necessary to further enhance delivery efficiency, reduce side effects, and expand the range of targetable tissues. Future research will aim to develop safer and more effective siRNA delivery methods, promoting broader clinical application of these innovative therapies.
To explore market dynamics and the competitive landscape in ASO, click on Patsnap Synapse.
For deeper insights into bio sequences and modifications, click on Patsnap Bio.
Refrence
- 1.Dong Y, Siegwart DJ, Anderson DG. Strategies, design, and chemistry in siRNA delivery systems. Adv Drug Deliv Rev. 2019 Apr;144:133-147. doi: 10.1016/j.addr.2019.05.004. Epub 2019 May 15. PMID: 31102606; PMCID: PMC6745264.
- 2.Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017 Mar;16(3):181-202. doi: 10.1038/nrd.2016.199. Epub 2016 Nov 3. Erratum in: Nat Rev Drug Discov. 2017 Jun;16(6):440. doi: 10.1038/nrd.2017.86. PMID: 27807347; PMCID: PMC5700751.
- 3.Springer AD, Dowdy SF. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018 Jun;28(3):109-118. doi: 10.1089/nat.2018.0736. Epub 2018 May 24. PMID: 29792572; PMCID: PMC5994659.