Rznomics Inc. Receives Australian Approval for Clinical Trial on RNA Editing Therapy in Retinitis Pigmentosa, Targeting RHO Gene
Rznomics Inc., a biopharmaceutical firm from South Korea focused on RNA-based gene therapy development, has reported that the Australian Therapeutic Goods Administration has provided Clinical Trial Notification to commence a Phase 1/2a clinical trial. This trial will assess RZ-004, a gene therapy candidate targeting autosomal dominant Retinitis pigmentosa associated with rhodopsin mutation.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 Retinitis pigmentosa is a genetically inherited retinal condition where photoreceptor cells in the retina deteriorate, resulting in blindness. Autosomal dominant Retinitis pigmentosa arises from numerous different genetic defects, with mutations in rhodopsin being the most frequent cause. Over 150 mutation sites in rhodopsin have been identified, complicating the treatment for rhodopsin-mediated autosomal dominant Retinitis pigmentosa.
Retinitis pigmentosa is a genetically inherited retinal condition where photoreceptor cells in the retina deteriorate, resulting in blindness. Autosomal dominant Retinitis pigmentosa arises from numerous different genetic defects, with mutations in rhodopsin being the most frequent cause. Over 150 mutation sites in rhodopsin have been identified, complicating the treatment for rhodopsin-mediated autosomal dominant Retinitis pigmentosa.
RZ-004 is an AAV vector that carries a rhodopsin RNA-targeting trans-splicing ribozyme along with wild-type rhodopsin. This vector selectively targets and reprograms the mutant pathogenic Rhodopsin mRNA into normal Rhodopsin mRNA, thereby producing a therapeutic effect. Because RZ-004 targets a conserved region upstream of the rhodopsin mutations, it has the potential to treat various mutations across different patients with a single therapy.
"The Clinical Trial Notification represents a significant step forward in addressing the substantial unmet medical need of autosomal dominant retinitis pigmentosa with rhodopsin mutations," said Dr. Seong-Wook Lee, Chief Executive Officer of Rznomics.
"Rznomics is dedicated to the development of innovative RNA-based biopharmaceuticals aimed at treating a range of rare and difficult-to-treat human diseases. We anticipate the commencement of the clinical trial to assess the safety and efficacy of RZ-004, providing a potential therapeutic option for patients affected by this condition," Dr. Seong-Wook Lee added.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
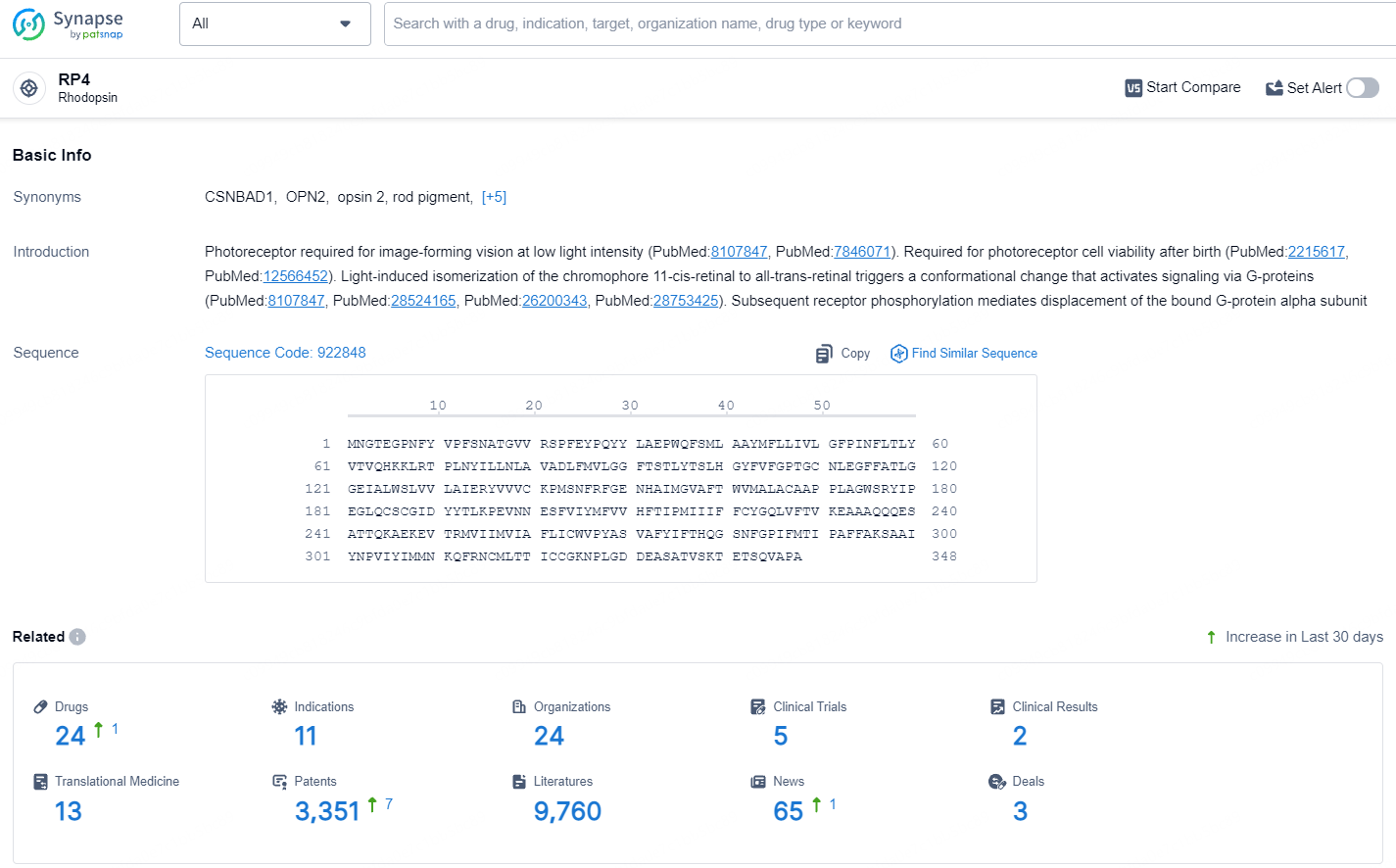
According to the data provided by the Synapse Database, As of July 18, 2024, there are 24 investigational drugs for the RP4 target, including 11 indications, 24 R&D institutions involved, with related clinical trials reaching 5, and as many as 3351 patents.
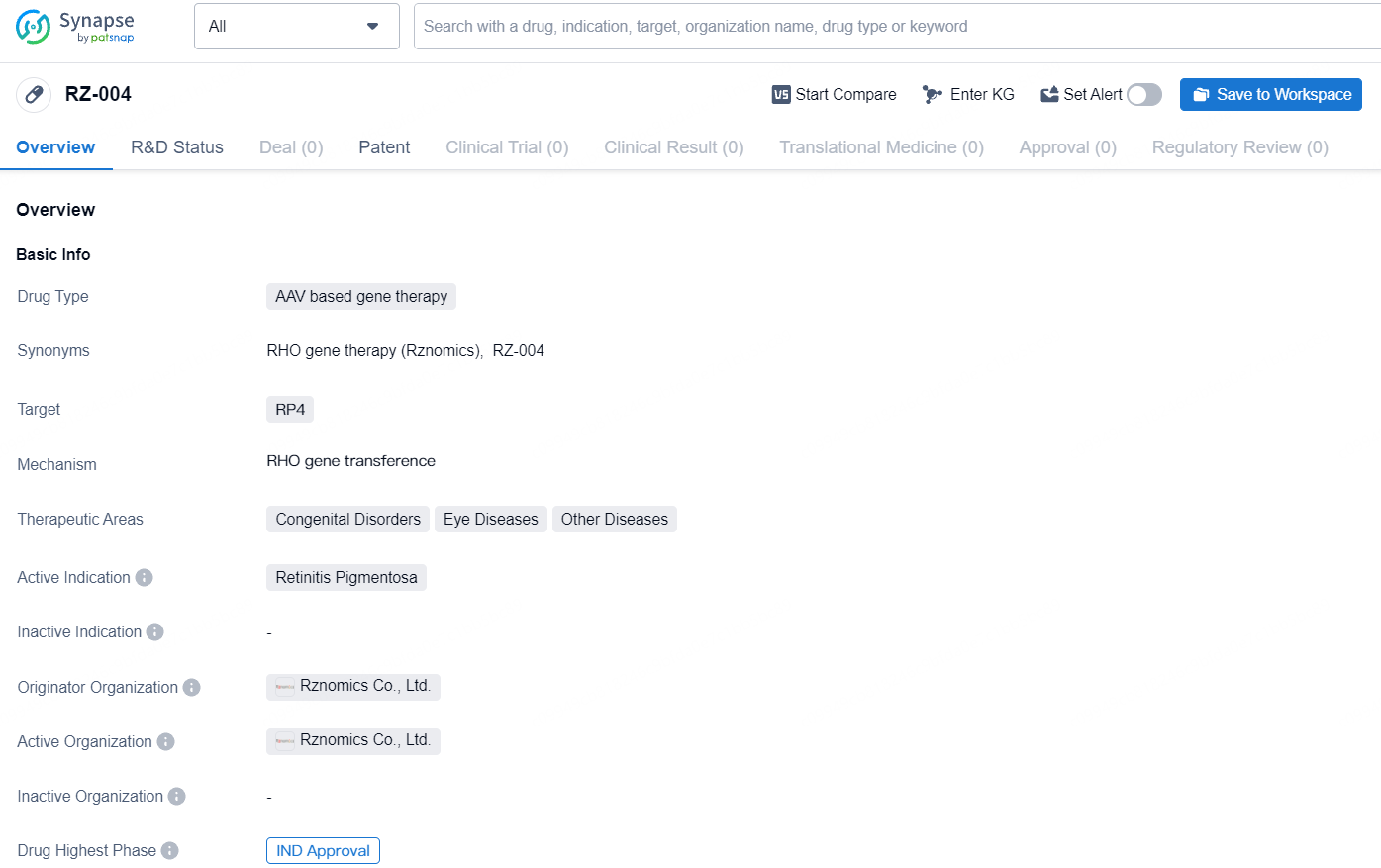
RZ-004 is an AAV based gene therapy that targets RP4, with its primary indication being Retinitis Pigmentosa. It falls under the therapeutic areas of Congenital Disorders, Eye Diseases, and Other Diseases. The IND approval positions Rznomics Co., Ltd. as a key player in the advancement of AAV based gene therapy and underscores the company's commitment to developing novel treatments for genetic and ocular disorders.