Scholar Rock Launches Phase 2 Apitegromab Trial and Unveils New SRK-439 Preclinical Obesity Data
Scholar Rock, a biopharmaceutical company in its late stages, dedicated to developing novel therapies for ailments such as spinal muscular atrophy, cardiometabolic disorders, and other severe diseases influenced by protein growth factors, has announced the commencement of the Phase 2 EMBRAZE proof-of-concept study. This study aims to evaluate the safety and effectiveness of apitegromab, a highly selective myostatin inhibitor, in maintaining lean muscle mass in obese individuals undergoing treatment with a GLP-1 receptor agonist.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The insights gained from this study will guide the advancement of SRK-439, an innovative investigational selective myostatin inhibitor tailored for managing cardiometabolic diseases like obesity. Additionally, the Company unveiled fresh preclinical results from a comparative study between SRK-439 and an anti-activin receptor II antibody. These findings highlight SRK-439’s promise as a leading candidate for preserving lean mass in patients using GLP-1 RAs. Mo Qatanani, Ph.D., Chief Scientific Officer, will present this data at Scholar Rock’s Investor Event, starting today at 8:30 a.m. ET in New York City.
“We are excited to have launched the EMBRAZE clinical trial ahead of schedule and to share new insights from our SRK-439 program, reinforcing our belief that selective latent myostatin inhibition offers benefits over less selective methods for safely and effectively maintaining lean muscle mass,” stated Jay Backstrom, M.D., MPH, President and Chief Executive Officer at Scholar Rock. “Selectivity is crucial for minimizing potential safety issues in this patient cohort, and we look forward to presenting more preclinical data at the American Diabetes Association 84th Scientific Sessions in June, supporting SRK-439’s potential as a best-in-class treatment.”
“The head-to-head comparison data indicate that selective myostatin inhibition alone is adequate to preserve lean mass against a backdrop of GLP-1 RA, in preclinical models. Coupled with the positive results at low doses of SRK-439, we believe our selective myostatin approach is the right potential solution for preserving lean muscle mass while avoiding the off-target effects seen in less selective treatments for this condition. This provides compelling evidence that SRK-439 could have a superior benefit/risk profile,” said Mo Qatanani, Ph.D., Chief Scientific Officer at Scholar Rock.
In January, Scholar Rock announced that the U.S. Food and Drug Administration had approved the Company’s Investigational New Drug application for EMBRAZE, a randomized, double-blind, placebo-controlled Phase 2 proof-of-concept trial. This trial will evaluate the efficacy, safety, and pharmacokinetics of apitegromab in adults with a body mass index (BMI) of over 27 or over 30 and who are taking a GLP-1 RA. The target enrollment for EMBRAZE is 100 participants aged 18-65 who are overweight or obese but do not have diabetes.
The presentation from Scholar Rock’s Investor Day will be available in the Investors and Media section of Scholar Rock’s website. The live webcast of the event can be accessed via the Investors & Media section of Scholar Rock’s website at http://investors.scholarrock.com. A recorded replay of the webcast will be available on the Company’s website for around 90 days following the presentations.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
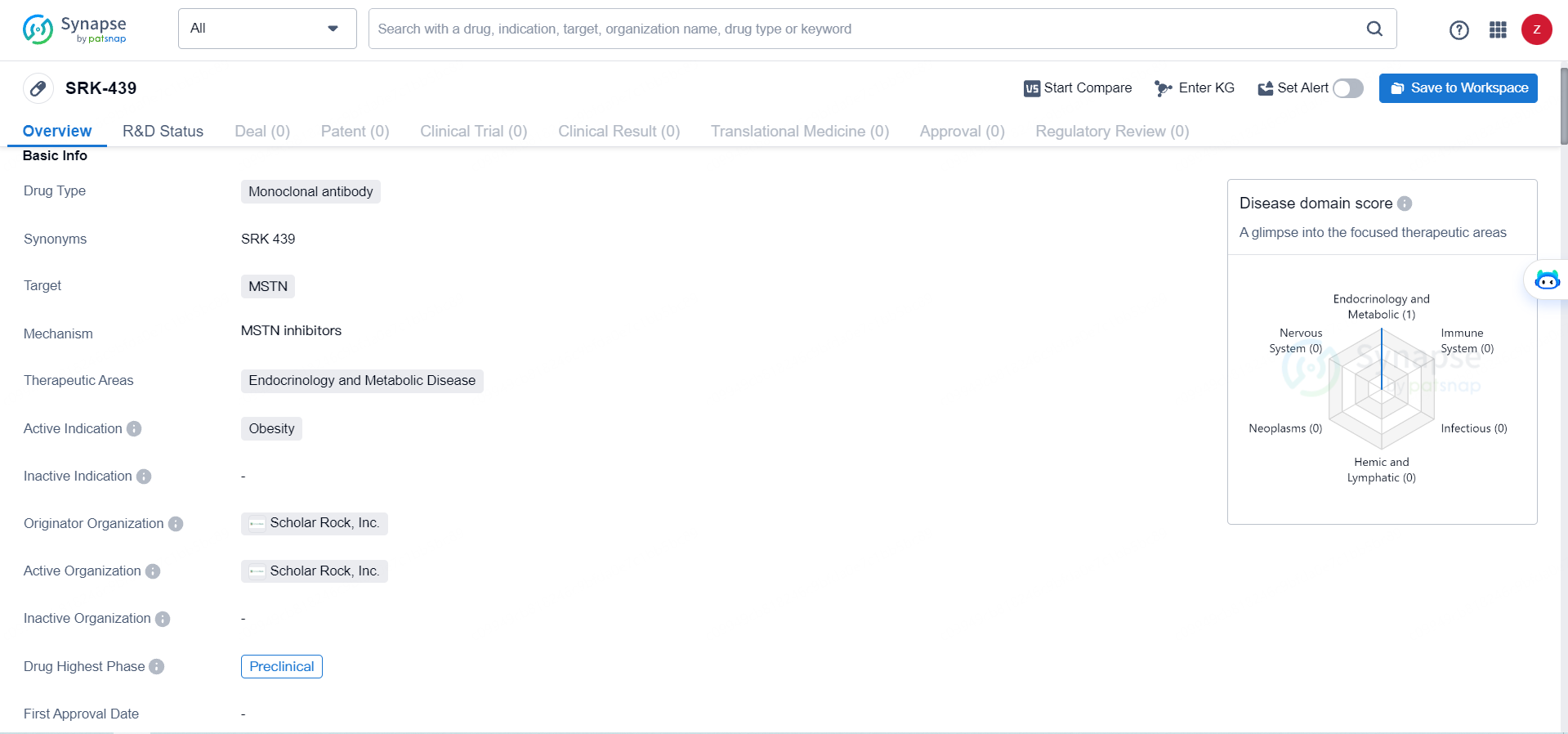
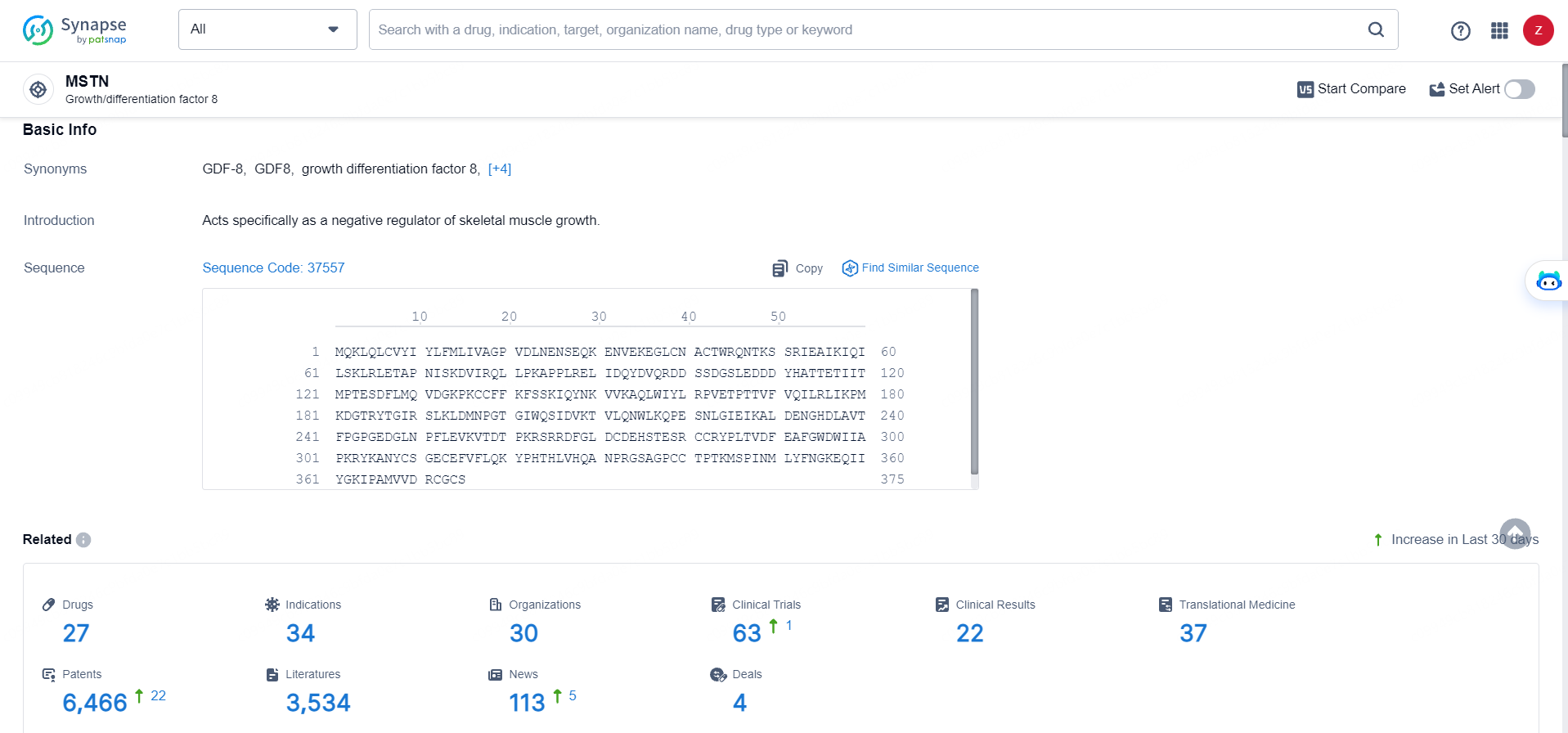
According to the data provided by the Synapse Database, As of May 28, 2024, there are 27 investigational drugs for the MSTN targets, including 34 indications, 30 R&D institutions involved, with related clinical trials reaching 63, and as many as 6466 patents.
SRK-439 targets MSTNand is intended for use in the therapeutic areas of endocrinology and metabolic disease, with a specific focus on treating obesity. While the drug is currently in the preclinical phase, its potential to address the unmet medical needs in obesity and related metabolic disorders makes it a promising candidate for further development and evaluation.