SciRhom Raises EUR 63 Million in Series A to Boost iRhom2 Therapy for Autoimmune Diseases
SciRhom GmbH, a biopharmaceutical firm at the forefront of creating first-in-class therapeutic iRhom2 antibodies, has declared the successful conclusion of a EUR 63 million Series A funding round. Leading the round were Andera Partners, Kurma Partners, Hadean Ventures, MIG Capital, and Wellington Partners. Bayern Kapital joined as a new investor, while existing investors such as High-Tech Gründerfonds (HTGF) and PhiFund Ventures from New York, USA, also participated.
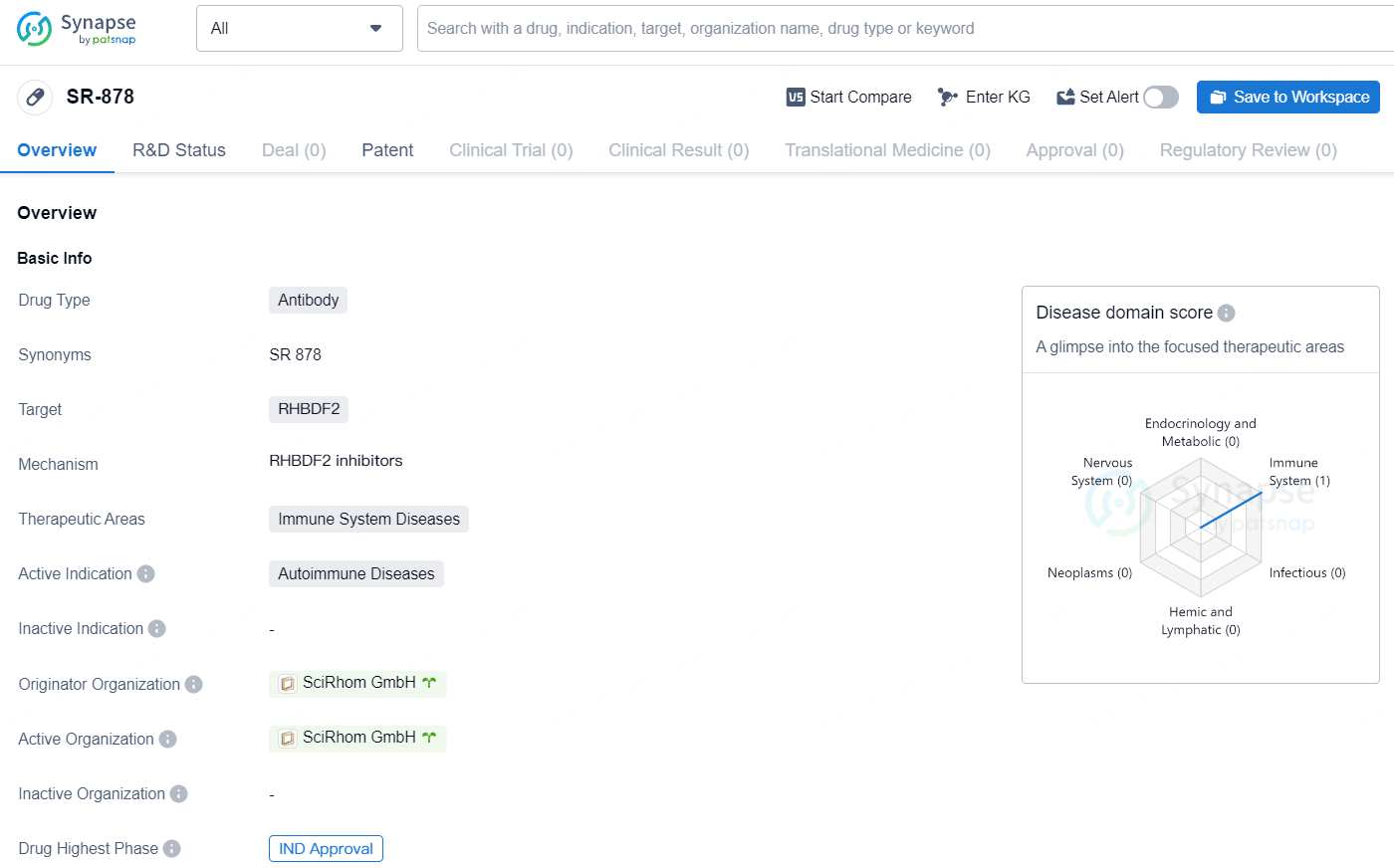
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The newly obtained funds will be allocated to expedite and extend the influence of the company's cutting-edge therapeutic approach in addressing autoimmune disorders. The initial clinical trial for SR-878, a highly specific monoclonal antibody targeting iRhom2, is anticipated to commence patient dosing in the latter half of 2024.
SciRhom was established with the objective of creating a new treatment paradigm for autoimmune diseases, and potentially other conditions, by selectively targeting TACE/ADAM17—a pivotal regulator of many autoimmune disease-related signaling pathways—via iRhom2. The SciRhom team has worked in close collaboration with co-founders Professor Carl Blobel and the Hospital for Special Surgery, the top academic medical center specialized in Rheumatology and musculoskeletal health globally. Prof. Blobel directs the Arthritis and Tissue Degeneration Program and has significantly contributed to the understanding of iRhom2’s role in regulating TACE/ADAM17 activity in inflammation and autoimmune diseases. SciRhom has developed its lead candidate, SR-878, to inhibit multiple pro-inflammatory and disease-driving pathways, such as TNF-alpha, IL-6R, and EGFR signaling, while maintaining other essential functions reliant on TACE/ADAM17.
This exclusive capability to target multiple cytokines and potentially induce immune tolerance by restoring beneficial TNFR2 signaling and regulatory T-cell expansion holds the promise of drastically impacting patients suffering from a broad array of autoimmune diseases. Additionally, the selective targeting of iRhom2 is expected to ensure a favorable safety profile.
"Since its inception, SciRhom has employed rigorous scientific methodologies to position itself as a leader in iRhom2-targeting biopharmaceuticals, establishing a comprehensive IND/CTA-enabling data, CMC package, and robust patent protections. The moment has come to escalate our innovative and potentially revolutionary therapeutic strategy to achieve clinical proof-of-concept and beyond, reaching patients who require improved autoimmune treatments,” said co-founder Dr. Jens Ruhe, Managing Director & COO of SciRhom.
Dr. Jan Poth, Managing Director & CEO of SciRhom, added: "We are thrilled to have garnered the support of such a prestigious international investor consortium and are grateful for the backing of our current shareholders during this pivotal phase of development. We eagerly anticipate collaborating with our new and existing partners and board members to deliver an innovative therapeutic solution for patients and address the critical necessity for more efficient and safe treatments for autoimmune disorders."
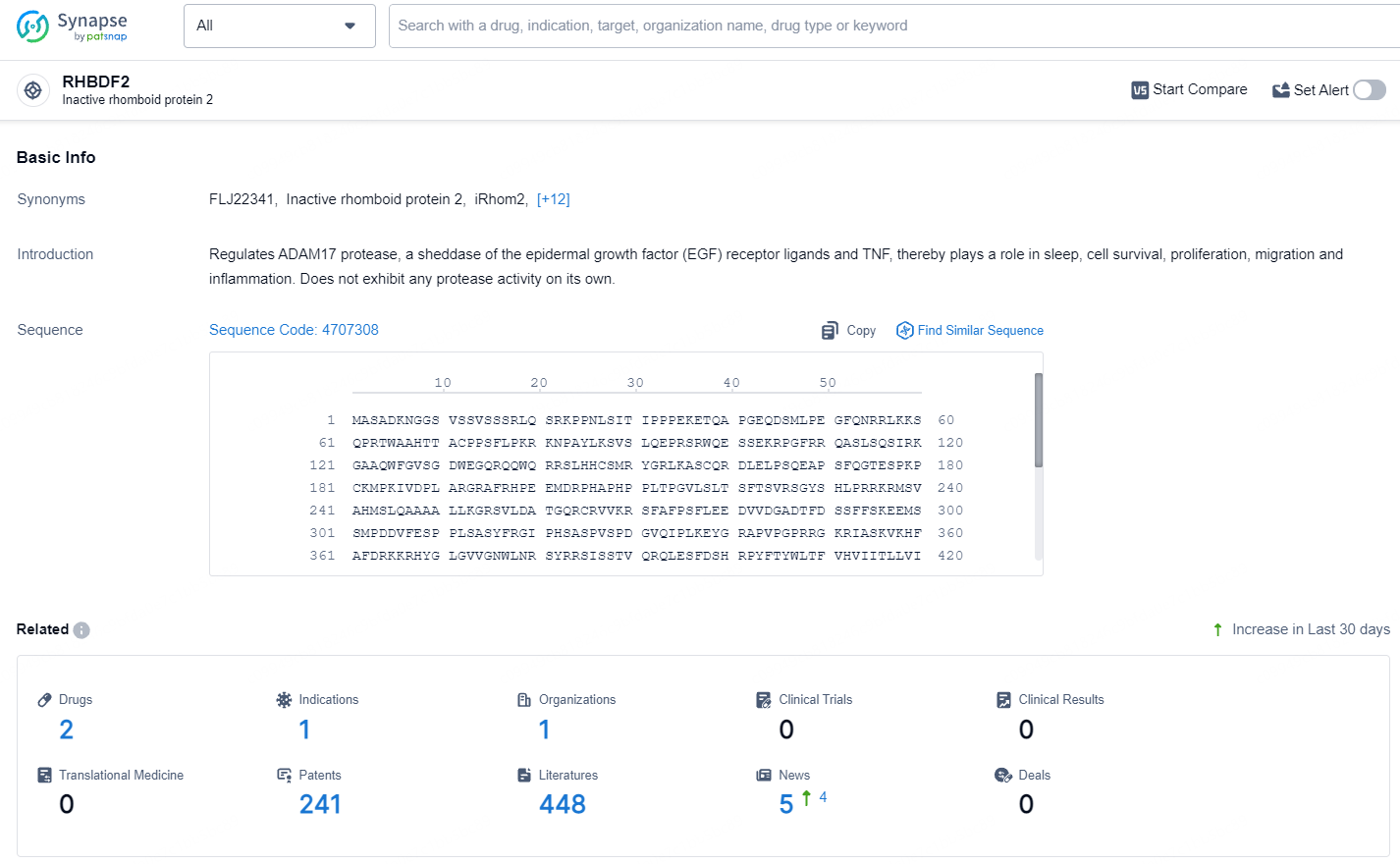
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 10, 2024, there are 2 investigational drugs for the RHBDF2 target, including 1 indication, 561 R&D institution involved, and as many as 241 patents.
SR-878 targets RHBDF2 and intended for the treatment of autoimmune diseases. With IND approval, the drug has advanced to the clinical development stage, indicating potential for addressing unmet medical needs in the field of biomedicine.