Seismic Therapeutic Presents New Preclinical Results for S-1117 in Immune Thrombocytopenia at 66th ASH Meeting
Seismic Therapeutic, Inc., a company specializing in machine learning immunology, has announced the unveiling of novel preclinical data for its pan-immunoglobulin G (IgG) sculpting enzyme candidate, S-1117, at the 66th American Society of Hematology (ASH) Annual Meeting, held from December 7-10 in San Diego, CA. The preclinical findings for S-1117 showed exceptional efficacy in both preventive and therapeutic mouse models of acute immune thrombocytopenia (ITP) when compared to a standard neonatal Fc receptor (FcRN) inhibitor treatment. ITP is an autoimmune disorder that leads the body to target its own platelets, causing a reduced platelet count and bleeding.
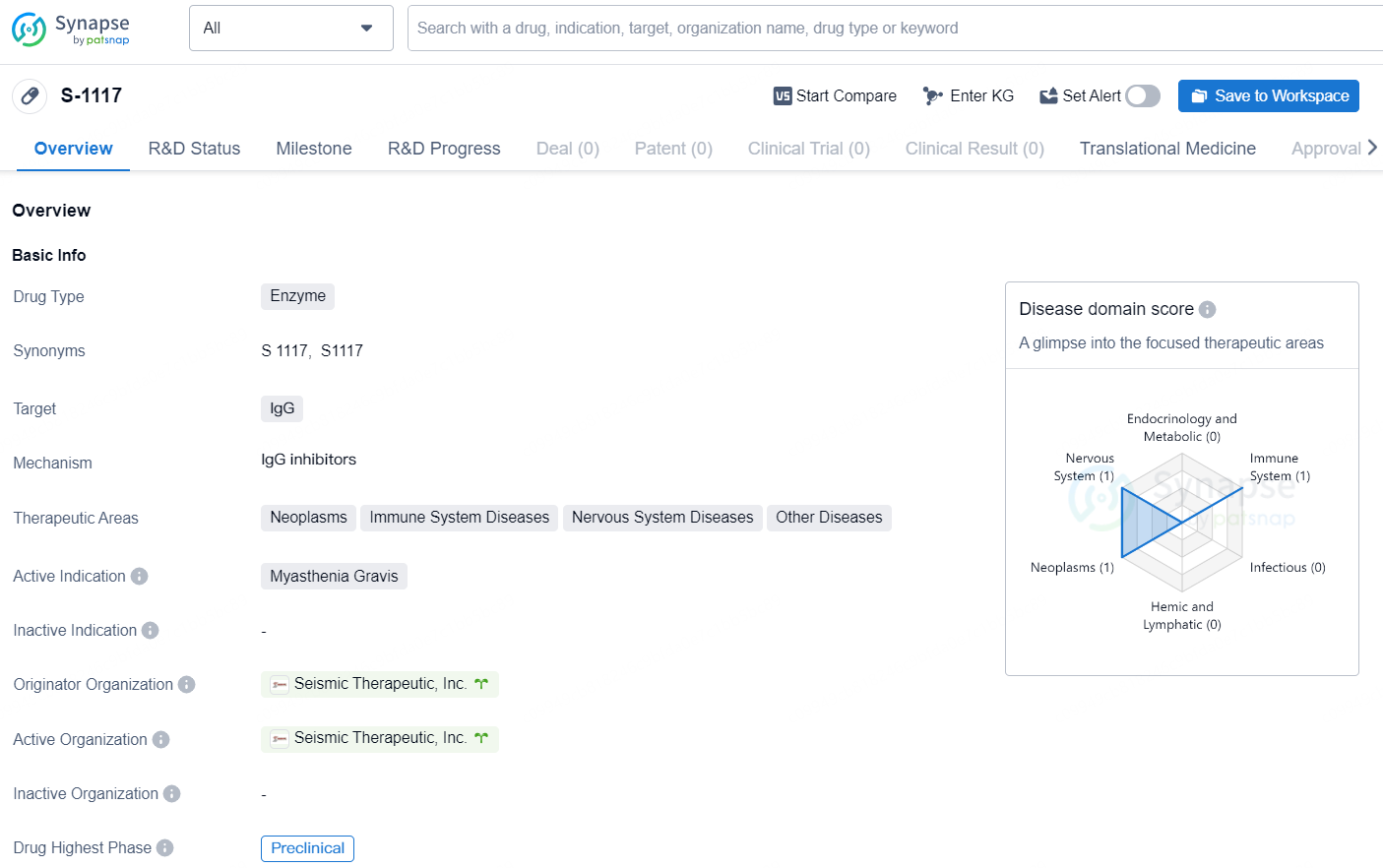
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
S-1117 is an innovative engineered Fc-fused pan-IgG protease designed to target IgG autoantibodies. This single molecule tackles multiple, clinically validated, independent pathogenic mechanisms, potentially leading to improved clinical outcomes in autoantibody-mediated conditions such as ITP and myasthenia gravis. Seismic intends to develop S-1117 for both chronic and acute treatment of IgG autoantibody-related diseases and anticipates initiating a Phase 1 clinical trial in healthy volunteers in the first half of 2025.
"Enzymatic IgG degradation by S-1117 represents a novel therapeutic strategy for addressing autoantibody-mediated diseases. The new preclinical ITP mouse model data further confirms the speed, depth, and extent of IgG reduction we can achieve in vivo with S-1117, as well as its superior activity in this context compared to a benchmark FcRN inhibitor," said John Sundy, MD, PhD, Chief Medical Officer and Head of R&D at Seismic Therapeutic. "These findings, along with the previously disclosed characteristics of S-1117, continue to underscore its potential as a rapidly acting, treat-to-target medication for both chronic and acute scenarios."
The S-1117 preclinical data presented at ASH 2024 expands on the recently showcased results from in vitro and in vivo studies, which indicated that S-1117 achieved profound, rapid, and sustained reduction of all IgG subclasses and was capable of directly cleaving circulating and immune complexed IgG, as well as the IgG B cell receptor expressed on memory B cells. S-1117 also holds the promise of a convenient, small volume, subcutaneous, self-administered treatment regimen administered every 4-6 weeks.
In the efficacy studies in animal models of ITP, acute ITP was induced in mice through injections of rabbit anti-mouse platelet serum (RAMS), and S-1117 was administered before or after RAMS injections in the prophylactic or therapeutic models, respectively. The new efficacy data presented at ASH 2024 includes the following key points:
- S-1117 showed superior efficacy in blocking platelet destruction compared to a benchmark FcRN inhibitor.
- In the prophylactic model, a single dose of S-1117 protected mice from antibody-mediated platelet destruction even with repeated RAMS dosing.
- In the therapeutic model, a single dose of S-1117 sped up and sustained platelet count recovery, maintaining efficacy even with repeated RAMS dosing.
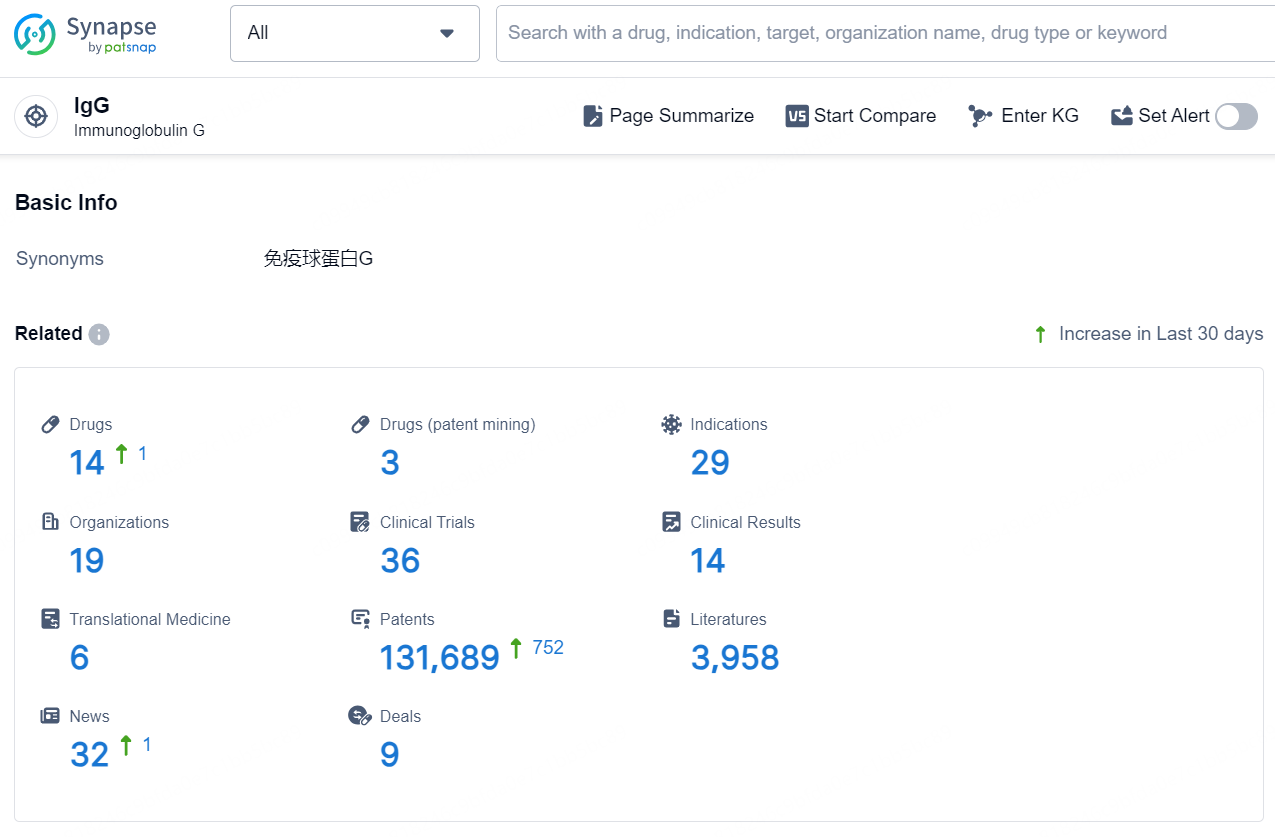
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 12, 2024, there are 14 investigational drugs for the IgG target, including 29 indications, 19 R&D institutions involved, with related clinical trials reaching 36, and as many as 131689 patents.
S-1117 is an enzyme drug that targets IgG and is being developed for the treatment of various therapeutic areas including neoplasms, immune system diseases, nervous system diseases, and other diseases. The active indication for S-1117 is Myasthenia Gravis.