selectION Reveals Encouraging Outcomes from Early-Stage Trial Testing si-544 on Individuals with Eczema
selectION, Inc., a company focused on the clinical development of innovative therapies for autoimmune conditions involving T-cells, has shared encouraging outcomes from its initial human Phase 1b study concerning its primary drug, si-544, targeted at individuals with atopic dermatitis. si-544 is designed as a peptide with enhanced selectivity aimed at obstructing the Kv1.3 ion channel.
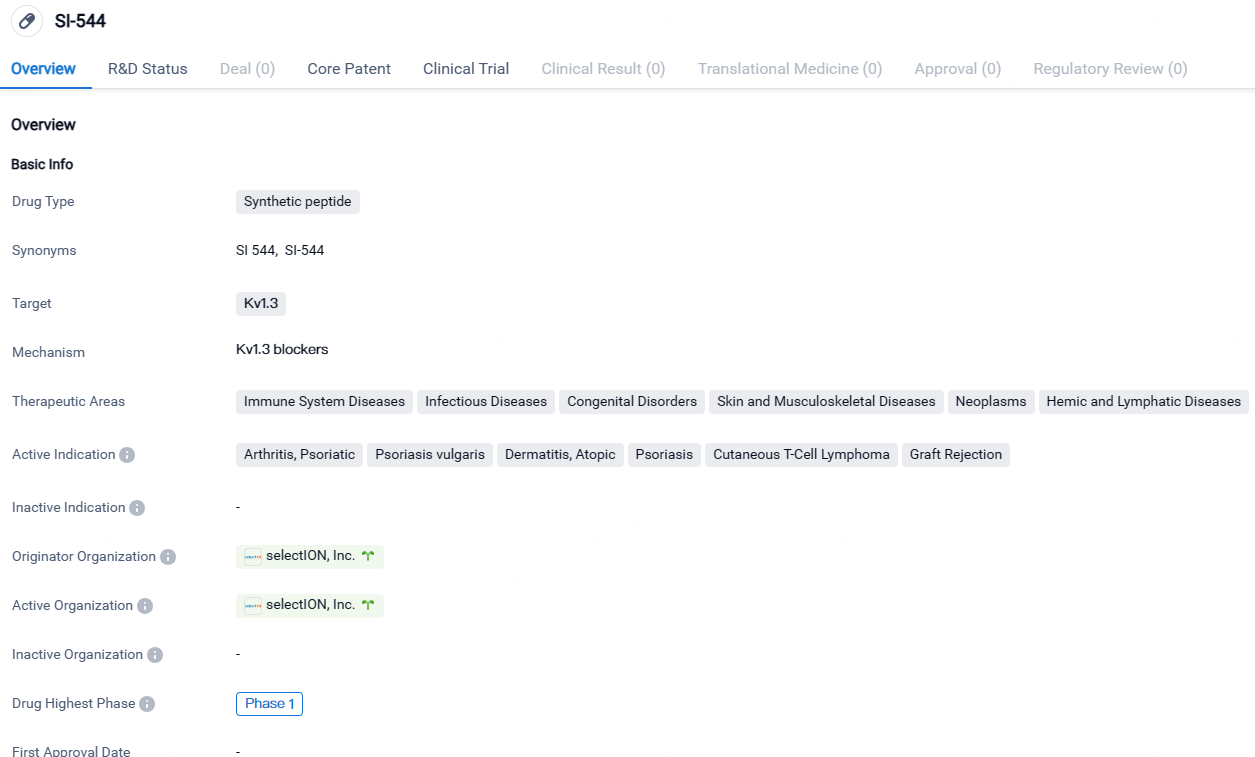
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The recently concluded Phase 1b trial, conducted across several centers and utilizing a double-blind, placebo-controlled design, involved participants with varying degrees of atopic dermatitis, from mild to severe. The primary purpose of the study was to assess the safety, tolerability, and preliminary efficacy of si-544.
si-544 showed good tolerability at even the highest dosage levels tested in both single and multiple ascending dose groups. Importantly, no safety concerns or dose-limiting toxicities emerged during the trial. Furthermore, the study did not reach safety and tolerability thresholds at the highest administered doses, indicating that the maximum tolerated dose could not be established. The trial noted no serious adverse events, nor were there any dose reductions or stoppages tied to adverse effects.
A significant 75% of participants who received the drug showed objective improvements clinically. Among these, 44% attained nearly clear or completely clear skin by the conclusion of the observation period. The therapeutic benefits continued throughout the full duration of the monitoring, even post-dosing.
“si-544 appears to hold promise for redefining the benchmarks for safety and tolerability in managing T-cell driven autoimmunity,” Antonius Schuh, PhD, Chairman and CEO of selectION, Inc., stated. “The trial's findings reveal an encouraging initial signal of efficacy. Furthermore, there was no evidence of immunosuppression under ongoing dosing. Plans are underway to further push si-544 into clinical development and assess its effects across additional autoimmune conditions.”
As the prime drug candidate from the Company, si-544 targets Kv1.3, an ion channel notably involved in the activation and growth of TEM cells. This specificity is believed to be at the forefront in terms of selectivity. TEM cells play a central role in several autoimmune diseases including atopic dermatitis, psoriasis, rheumatoid arthritis, and multiple sclerosis, and are also linked to certain rare cancers such as lymphomas.
In its Phase 1b clinical trial targeting atopic dermatitis patients, si-544 maintained an impressive safety and tolerability profile while the initial efficacy signals were positive. Earlier, si-544 had also shown promising efficacy against T-cell driven devises in both animal studies and human T-cell models.
si-544 operates as a highly effective immuno-selective agent aimed at managing significant unaddressed medical needs by selectively blocking and eliminating disease-specific, persistently active TEM cells, yet preserving overall immune functionality.
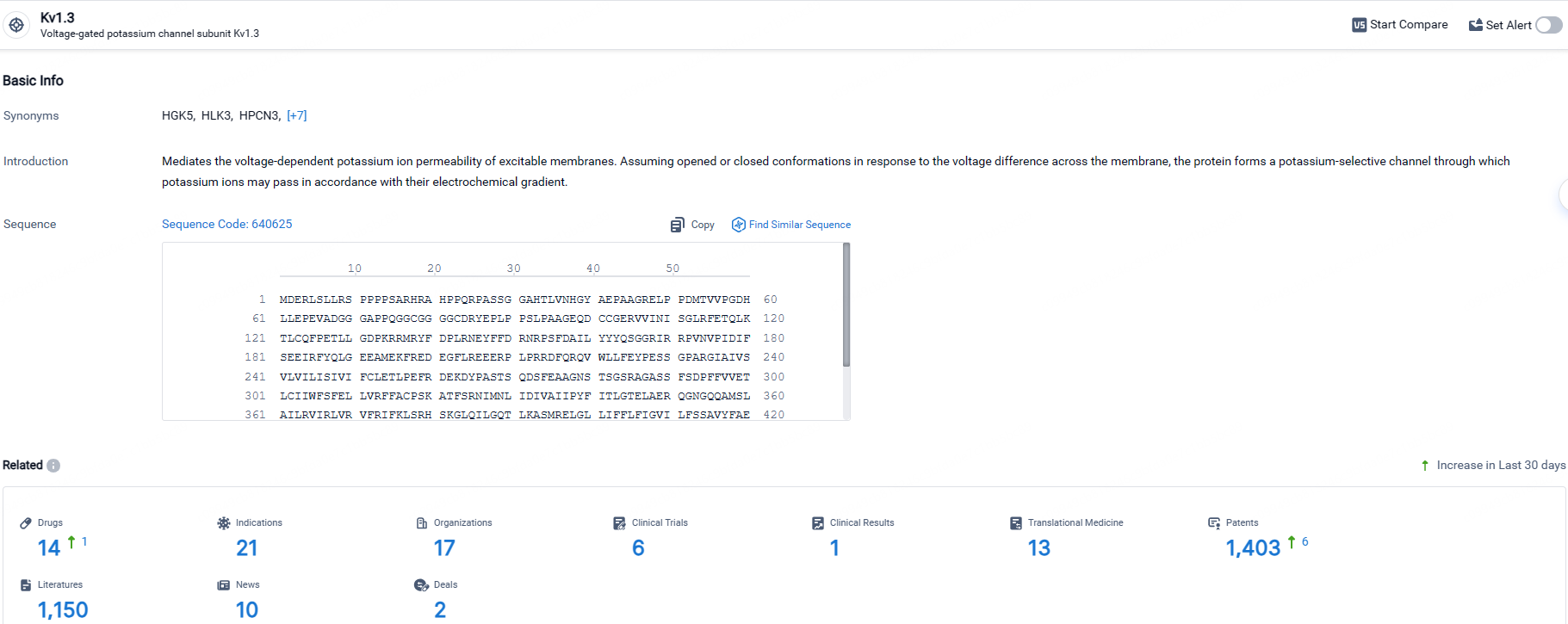
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 30, 2024, there are 14 investigational drugs for the Kv1.3 target, including 21 indications, 17 R&D institutions involved, with related clinical trials reaching 6, and as many as 1403 patents.
SI-544 shows promise in treating various immune system diseases, infectious diseases, congenital disorders, skin and musculoskeletal diseases, neoplasms, and hemic and lymphatic diseases. The drug is currently in Phase 1 of clinical trials, indicating that further research is being conducted to evaluate its safety and potential efficacy.