Senaparib: A Next-Generation PARP1/2 Inhibitor Approved in China
Senaparib is a next-generation, highly potent PARP1/2 inhibitor developed by IMPACT Therapeutics. It received market approval from the National Medical Products Administration (NMPA) on January 16, 2025. The primary mechanism of action of Senaparib involves targeting and inhibiting poly(ADP-ribose) polymerase (PARP), specifically the PARP1 and PARP2 isoforms, to prevent cancer cells from repairing DNA damage, ultimately leading to tumor cell death.
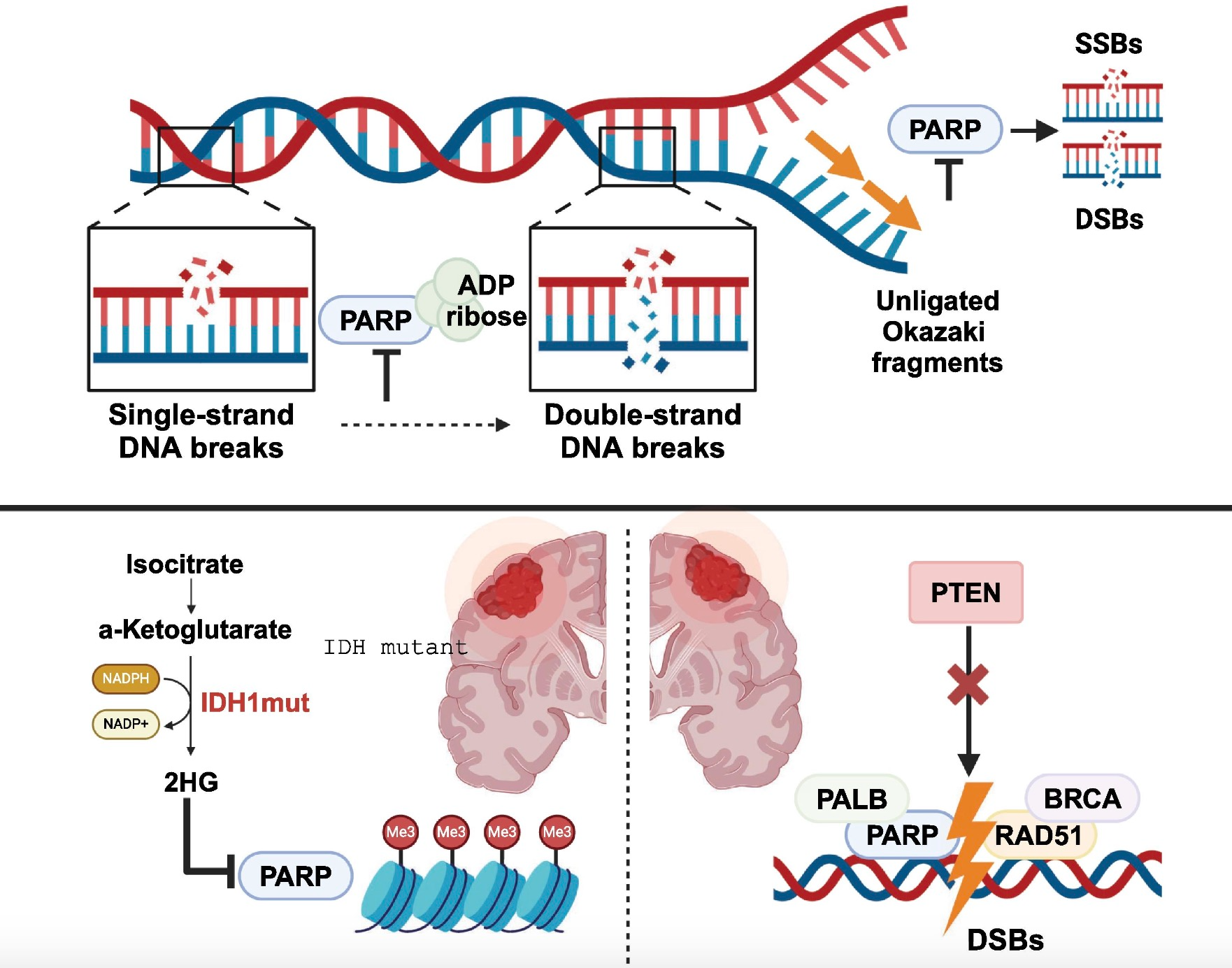
Due to its unique molecular structure, Senaparib has emerged as a potential best-in-class (BIC) drug. In the pivotal Phase III FLAMES trial, Senaparib was evaluated as a maintenance therapy following a complete response (CR) or partial response (PR) to first-line platinum-based chemotherapy in patients with advanced epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer.
The trial results were highly encouraging, demonstrating that Senaparib significantly prolonged progression-free survival (PFS) compared to placebo, with a hazard ratio (HR) of 0.43. Importantly, this clinical benefit was independent of BRCA mutation status, meaning that Senaparib provided substantial therapeutic efficacy regardless of whether patients had BRCA mutations. This finding is particularly significant as it broadens the eligible patient population for the treatment.
The FLAMES study also highlighted the favorable safety profile of Senaparib. Compared to other PARP inhibitors, Senaparib exhibited lower non-hematologic toxicity, with a permanent discontinuation rate due to adverse events of only 4.4%. This data underscores the excellent tolerability of Senaparib, allowing patients to stay on treatment longer, which may lead to better long-term clinical outcomes.
Beyond its clinical success, Senaparib has also gained recognition in the capital markets. IMPACT Therapeutics successfully raised RMB 250 million in a Series D++ financing round, with the proceeds designated for Senaparib’s commercialization and the global clinical development of multiple compounds. This financial backing enhances the potential for Senaparib’s broader international adoption and expansion.
Additionally, Huadong Medicine has secured exclusive marketing rights for Senaparib in mainland China. This strategic partnership is expected to further accelerate Senaparib’s market penetration in China, ensuring that more patients benefit from this innovative therapy.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference:
- 1. Cella E, Bosio A, Persico P, Caccese M, Padovan M, Losurdo A, Maccari M, Cerretti G, Ius T, Minniti G, Idbaih A, Sanai N, Weller M, Preusser M, Simonelli M, Lombardi G. PARP inhibitors in gliomas: Mechanisms of action, current trends and future perspectives. Cancer Treat Rev. 2024 Dec;131:102850. doi: 10.1016/j.ctrv.2024.102850. Epub 2024 Nov 5.