Simcha Therapeutics has launched two clinical trials to investigate ST-067 for blood disorders
Simcha Therapeutics, an innovative immunobiology firm focused on developing first-in-class cytokine therapies for cancer treatment, has announced the initiation of two clinical trials evaluating ST-067, their decoy-resistant IL-18, for hematological conditions. One trial (ClinicalTrials.gov ID NCT06492707) will investigate ST-067 in individuals diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS), while the second trial (ClinicalTrials.gov ID NCT06588660) will explore the use of ST-067 in conjunction with teclistamab (TECVAYLI®) in patients suffering from multiple myeloma.
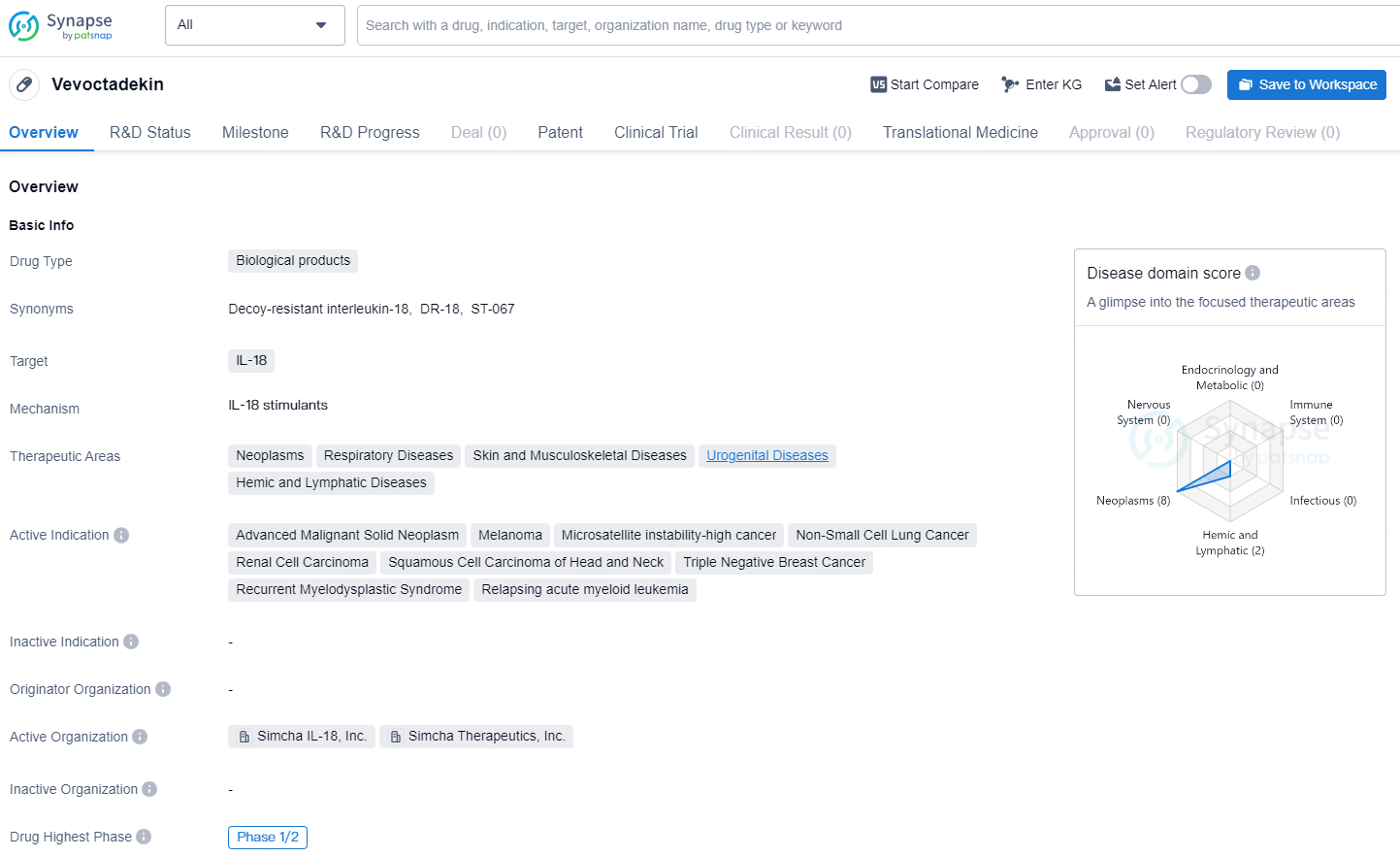
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Sanuj Ravindran, M.D., the CEO of Simcha, stated, “These two research initiatives are poised to quickly yield clinical proof-of-concept data in areas where there is a pressing demand for improved treatments, presenting an exciting chance for us to investigate further therapeutic domains that may significantly benefit from IL-18 treatment. The emerging data, including findings that we will present in our preclinical poster at ASH, indicate promising proof-of-concept signals regarding the potential of IL-18 in treating various hematological malignancies. Therefore, we are eager to collaborate with leading experts in hematologic cancers for these trials.”
The study focusing on AML/MDS, led by principal investigator Elizabeth Krakow, M.D. from Fred Hutch Cancer Center, is currently enrolling participants aged 18 and older who are experiencing persistent or recurrent AML or MDS post-hematopoietic cell transplantation (HCT), commonly known as a bone marrow transplant. While HCT remains the only curative option for the majority of AML or MDS cases, approximately one-third of patients will relapse, which is the primary cause of mortality following HCT.
This Phase 1, open-label, dose-escalation trial will mainly investigate dose-limiting toxicities and the number of subjects who complete four consecutive weeks of treatment with ST-067. Secondary measures will include response rates, overall survival, and the capability to induce graft-versus-leukemia effects without triggering graft-versus-host disease.
Another trial examines the use of ST-067 in conjunction with teclistamab, a bi-specific antibody that engages T cells, in patients aged 18 and older with relapsed or refractory multiple myeloma. This study is being conducted under the supervision of Rahul Banerjee, M.D., at the University of Washington and Fred Hutch Cancer Center. The open-label Phase 1b trial will primarily evaluate the dose-limiting toxicities associated with ST-067, both alone and in combination with teclistamab, alongside determining optimal dosages and monitoring for adverse events. Secondary endpoints will assess overall response rate, duration of response, progression-free survival, overall survival, and measurable residual disease negativity.
T-cell directed therapies, including teclistamab, other bi-specific antibodies, and CAR T therapies, have emerged as standard treatments for relapsed or refractory multiple myeloma. Nevertheless, these options are not curative, as some patients may not respond to teclistamab, and relapses can occur even in those who do. The rationale behind the multiple myeloma study is that combining ST-067 with teclistamab may enhance T-cell fitness and persistence, potentially increasing the proportion of patients who respond to teclistamab and extending the duration of their response.
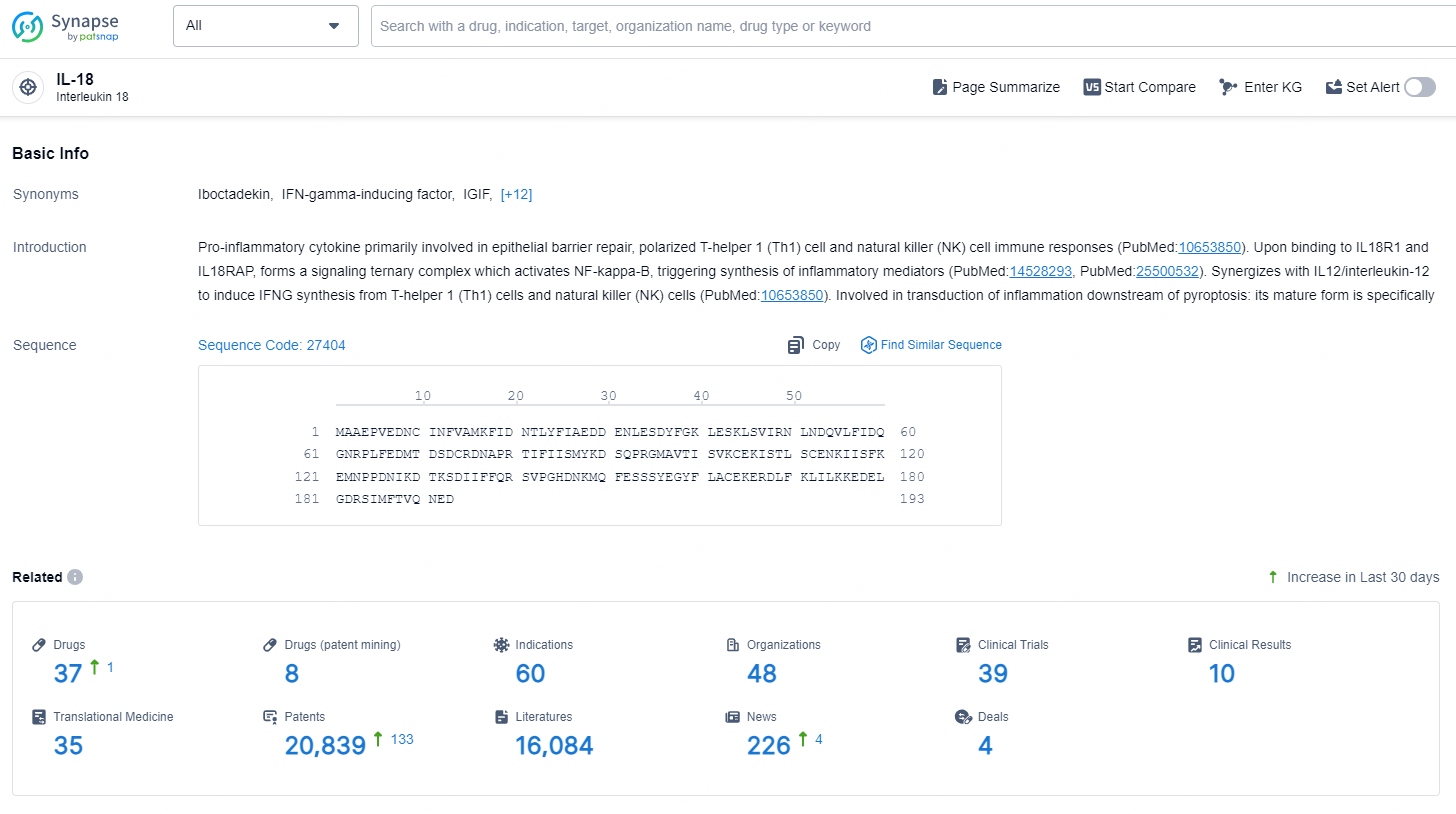
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 10, 2024, there are 37 investigational drugs for the IL-18 target, including 60 indications, 48 R&D institutions involved, with related clinical trials reaching 39, and as many as 20839 patents.
Vevoctadekin is a biological product that targets the inflammatory cytokine IL-18. It is being developed for the treatment of a wide range of therapeutic areas including neoplasms, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, and hemic and lymphatic diseases.