Sobi Files FDA Application for SEL-212, a Chronic Refractory Gout Treatment
Sobi disclosed the commencement of a rolling Biologics License Application to the U.S. Food and Drug Administration for SEL-212. This submission is predicated on the outcomes from the pivotal studies DISSOLVE I and II. SEL-212 is a novel biologic therapy being developed to treat chronic refractory gout, a severe condition marked by the continuous and painful accumulation of urate crystals in the joints.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
This major step follows the FDA's Fast Track status granted to SEL-212 in March 2024, highlighting the critical need for new treatments for chronic refractory gout patients. The FDA's Fast Track initiative aims to speed up the development and review processes for drugs that address serious conditions and fulfill an unmet medical requirement.
“We are excited to begin the rolling submission of the BLA for SEL-212, which brings Sobi closer to offering a promising new treatment option for individuals with chronic refractory gout,” stated Lydia Abad-Franch, MD, MBA, Head of Research, Development, and Medical Affairs, and Chief Medical Officer at Sobi.
“The Fast Track status validates the earlier phase 3 clinical findings for SEL-212, emphasizing the urgent need for innovative therapies in this field and reinforcing our dedication to improving the lives of patients with rare diseases,” Lydia Abad-Franch further commented.
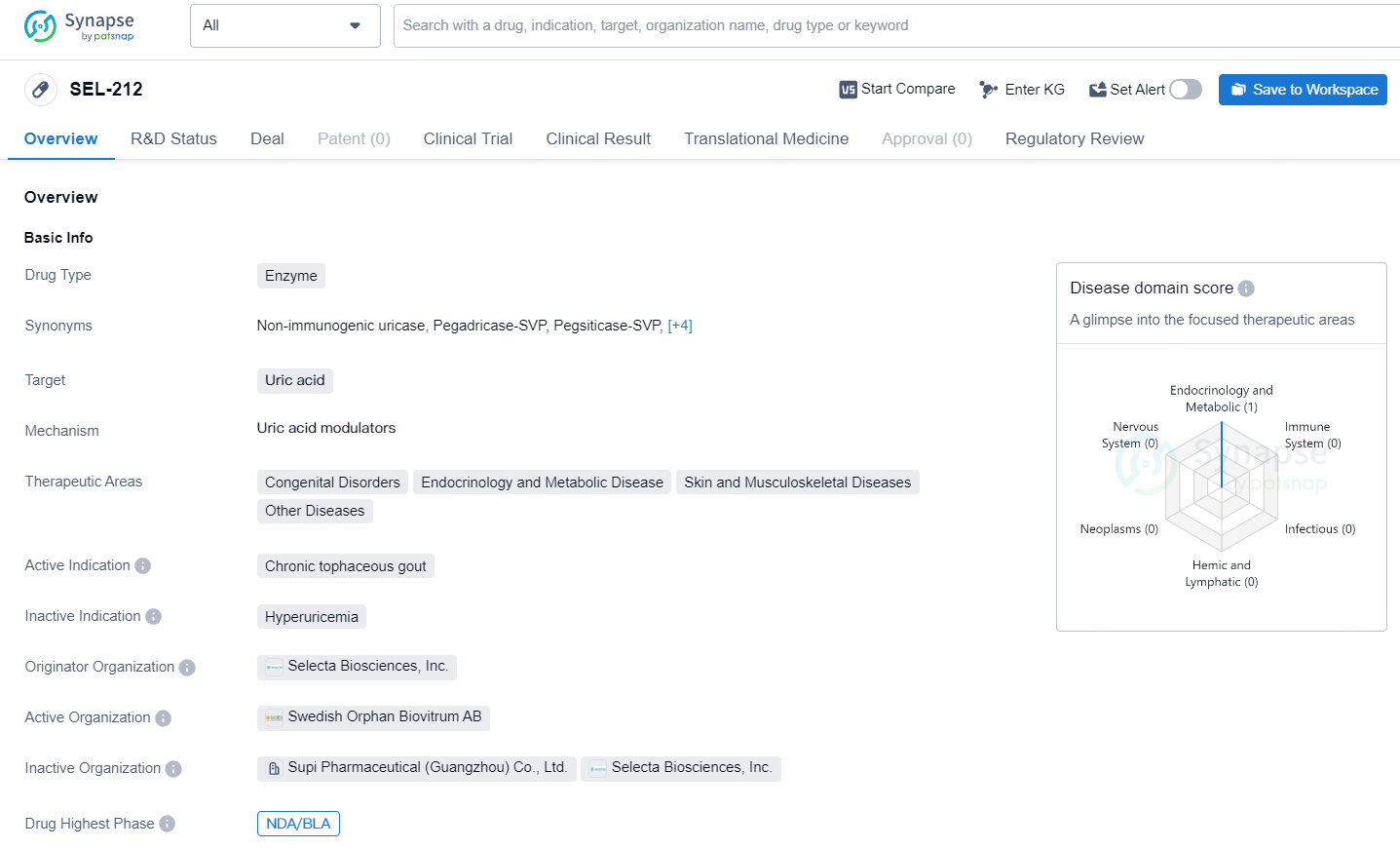
SEL-212 is an investigational combination medicine aimed at lowering serum urate levels in patients with chronic refractory gout, potentially averting the buildup of harmful tissue urate deposits which, if untreated, can cause gout flares and joint deformities. Sobi acquired the rights for SEL-212 from Selecta Biosciences in June 2020, overseeing its development, regulatory, and commercial efforts outside China.
SEL-212 comprises pegadricase, Selecta’s exclusive pegylated uricase, administered alongside ImmTOR™, designed to minimize the creation of anti-drug antibodies (ADAs). The development of ADAs, triggered by adverse immune reactions to biologic drugs, diminishes their effectiveness and tolerability, presenting a challenge across various therapeutic areas and disease conditions, including chronic refractory gout.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
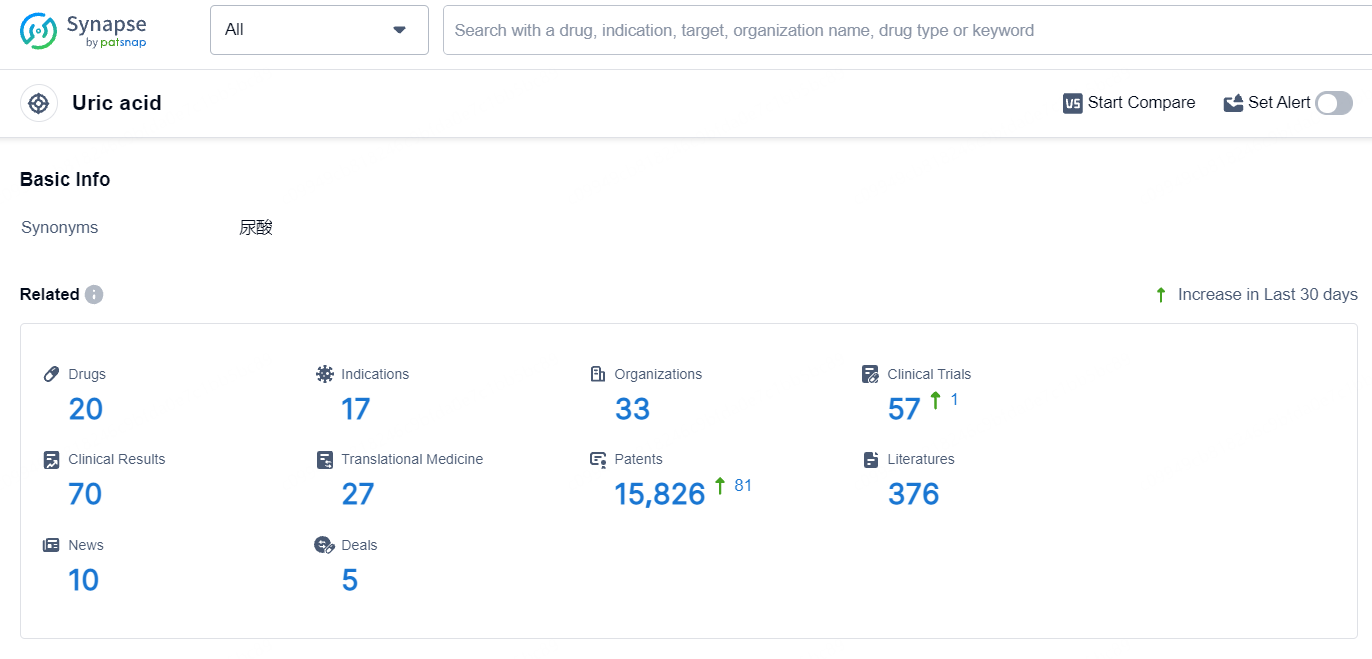
According to the data provided by the Synapse Database, As of July 8, 2024, there are 20 investigational drugs for the uric acid target, including 17 indications, 33 R&D institutions involved, with related clinical trials reaching 57, and as many as 15826 patents.
SEL-212 targets uric acid and is intended for the treatment of chronic tophaceous gout.SEL-212 is an enzyme-based drug developed by Selecta Biosciences, Inc. that targets uric acid and is intended for the treatment of chronic tophaceous gout. It has reached the highest phase of development globally, with a regulatory designation of Fast Track, indicating its potential to address unmet medical need.