Sonnet BioTherapeutics Announces Collaboration on Funded Phase 1/2a Trial of SON-1210 with Chemotherapy for Pancreatic Cancer
Sonnet BioTherapeutics Holdings, Inc. (referred to as the “Company” or “Sonnet”) (NASDAQ: SONN), a clinical-stage organization focused on creating targeted immunotherapeutic drugs, announced the signing of a Master Clinical Collaboration Agreement (the “Agreement”) with the Sarcoma Oncology Center. This collaboration aims to advance the development of SON-1210, Sonnet’s proprietary bifunctional fusion of human Interleukins 12 (IL-12) and 15 (IL-15). SON-1210, engineered using Sonnet’s Fully Human Albumin Binding (FHAB®) platform, will be explored in combination with chemotherapy for treating metastatic pancreatic cancer.
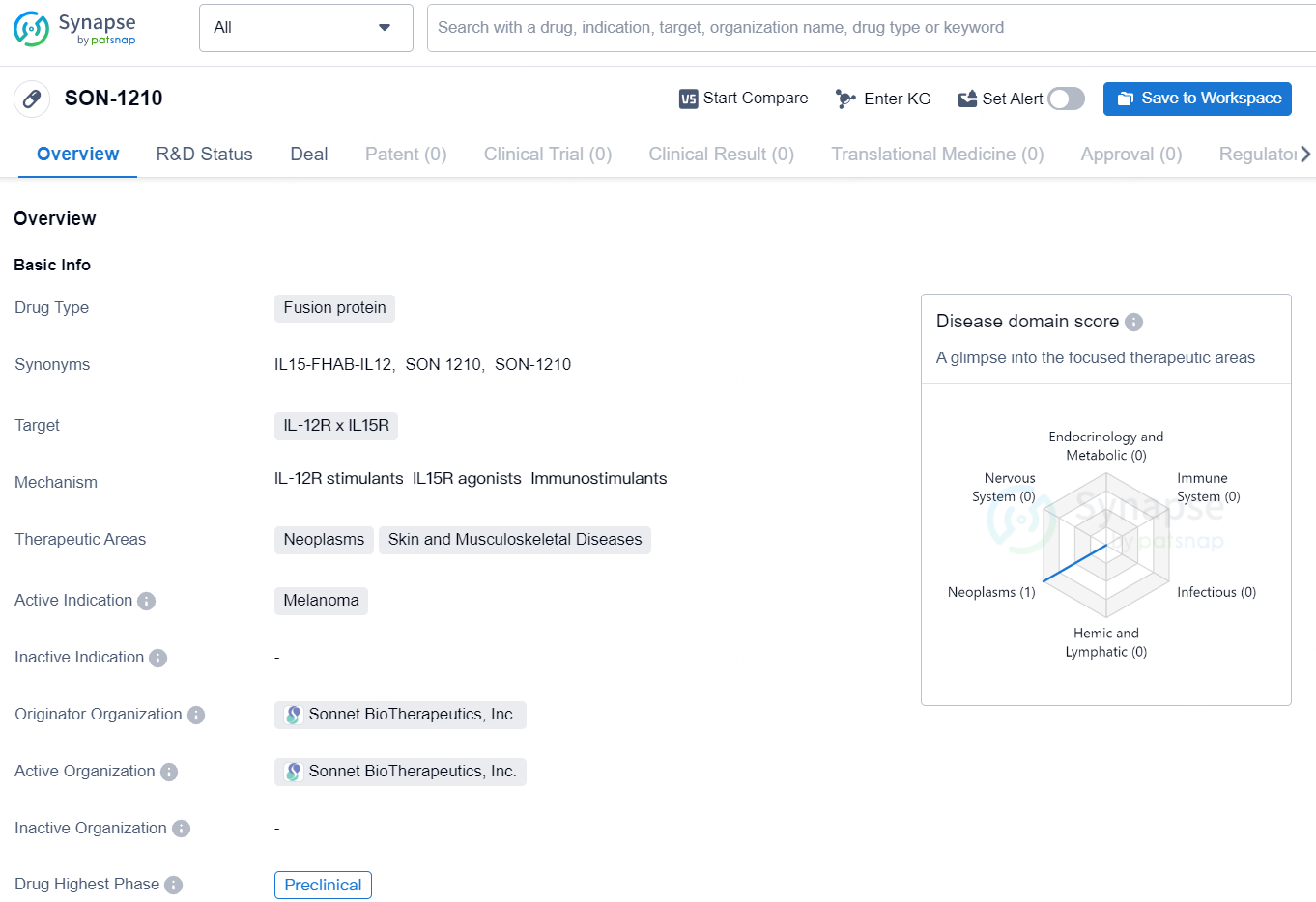
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are delighted to form this strategic development partnership to progress SON-1210 with this esteemed institution, within a program that we believe addresses a critical unmet need. We anticipate that this developmental strategy will yield invaluable insights into a high-priority cancer indication, contributing to our growing data repository. We believe this will potentially enhance the value of our platform and aid in conserving our financial resources," stated Pankaj Mohan, Ph.D., Founder and CEO of Sonnet.
Dr. Hendifar, an acclaimed physician involved in developing novel therapies for pancreatic cancer and neuroendocrine tumors, as well as Associate Professor of Medicine, Medical Director of Pancreatic Cancer, Co-Director of Hematology-Oncology Fellowship Program, and Medical Director of the Gastrointestinal Oncology Disease Research Group at Cedars-Sinai, remarked, "The global incidence of pancreatic cancer is rising, and progress in care standards beyond conventional chemotherapies has been limited. We believe that an innovative immunotherapy like SON-1210 presents an exciting chance to better patient outcomes."
Under the terms of the Agreement, the Sarcoma Oncology Center, directed by Dr. Sant Chawla, and in collaboration with Sonnet, will develop a protocol and conduct a Phase 1/2a investigator-initiated clinical trial to evaluate SON-1210 in combination with multiple chemotherapy agents including but not limited to liposomal irinotecan, 5-fluorouracil/leucovorin, and oxaliplatin (“NALIRIFOX”) for treating metastatic pancreatic cancer. NALIRIFOX has received U.S. FDA approval for the treatment of metastatic pancreatic cancer in both front-line and refractory settings. Sonnet will provide SON-1210 and support services for the planned Phase 1/2a trial.
"We are thrilled to embark on this partnership with Sonnet. Cancer cells avidly absorb albumin and albumin-bound medications, which are preferentially taken up by these cells,” commented Dr. Chawla, a prominent expert in medical treatment and research for bone and soft-tissue sarcomas. “This is a validated approach for enhancing efficacy while minimizing toxicity, and currently, there are no approved immunotherapies for pancreatic cancer. SON-1210’s dual IL-12 and IL-15 strategy builds on the success of SON-1010 in extending cytokine half-life and converting cold tumors to hot, which we are also studying at our center.”
John Cini, Ph.D., Chief Scientific Officer and Co-Founder of Sonnet, concluded, "Cytokine-based clinical trials have historically faced limitations due to their brief half-lives, the inability to directly target cancer cells, and high adverse event rates. Sonnet’s fully human albumin-binding platform is uniquely designed to address these challenges. Based on our FHAB construct, we have demonstrated that tumor-targeting and retention can be increased by 4-5 times compared to non-modified cytokines. We are targeting pancreatic cancer, a field with significant unmet needs, and high expression of FcRn and GP60 receptors, as well as the SPARC complex. SON-1210, our bifunctional IL-12/IL-15 candidate, has exhibited enhanced immune activity and persistence in preclinical models, and we are enthusiastic about commencing human studies with this distinctive candidate. SON-1210 may work synergistically with NALIRIFOX, the first newly approved chemotherapy for front-line pancreatic cancer in over a decade."
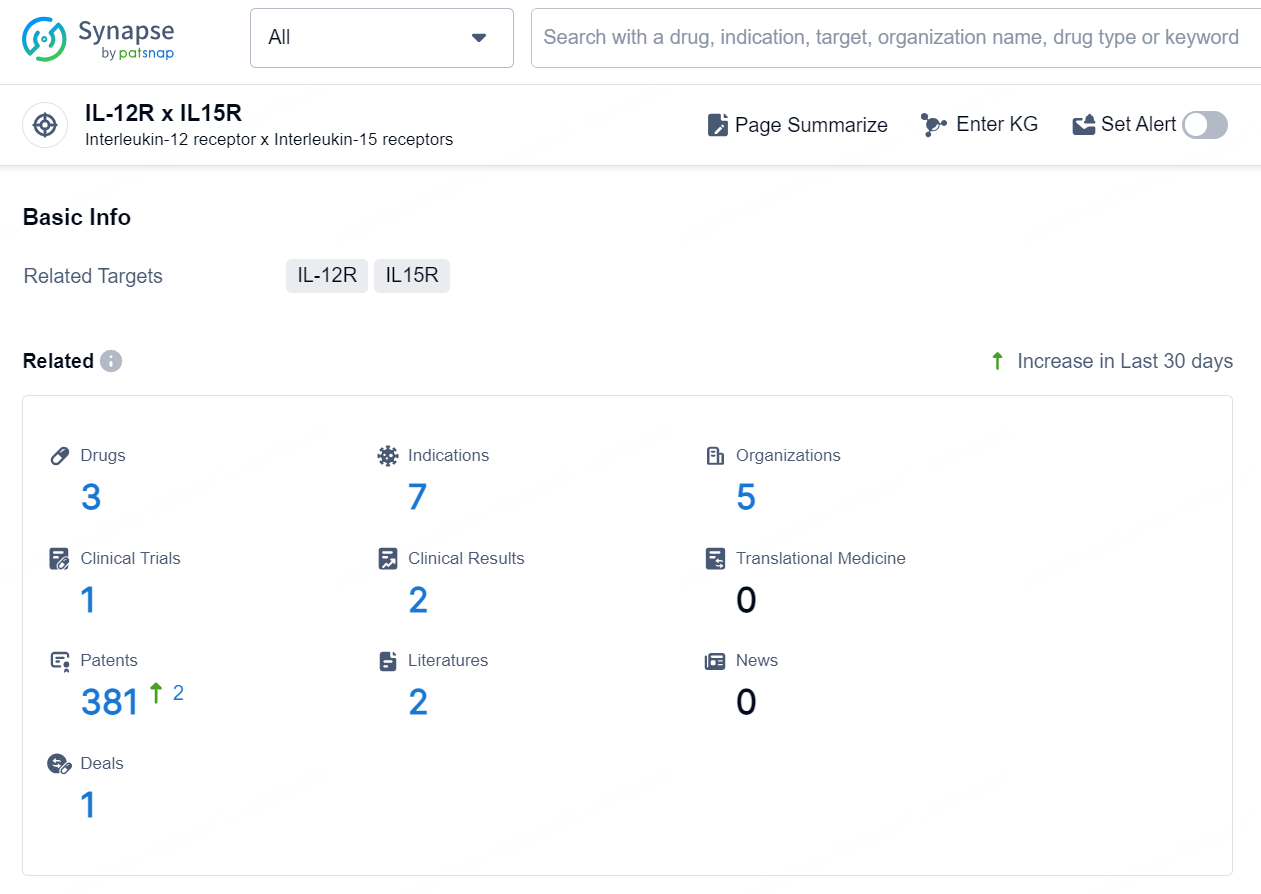
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 22, 2024, there are 3 investigational drugs for the IL-12R x IL15R targets, including 7 indications, 5 R&D institutions involved, with related clinical trial reaching 1, and as many as 381 patents.
SON-1210 is an immunotherapeutic bifunctional drug candidate that links unmodified single-chain human IL-12 and human IL-15 with the albumin-binding domain of the single-chain antibody fragment FHAB separating the two cytokines with linkers to avoid steric hindrance. The FHAB single chain was selected to bind well at normal pH, as well as at an acidic pH that is typically found in the tumor microenvironment (TME). The FHAB technology targets tumor and lymphatic tissue, providing a mechanism for dose-sparing, enhanced PK, and an opportunity to improve the safety and efficacy profile of not only IL-12 and IL-15, but a variety of other potent immunomodulators using the platform.