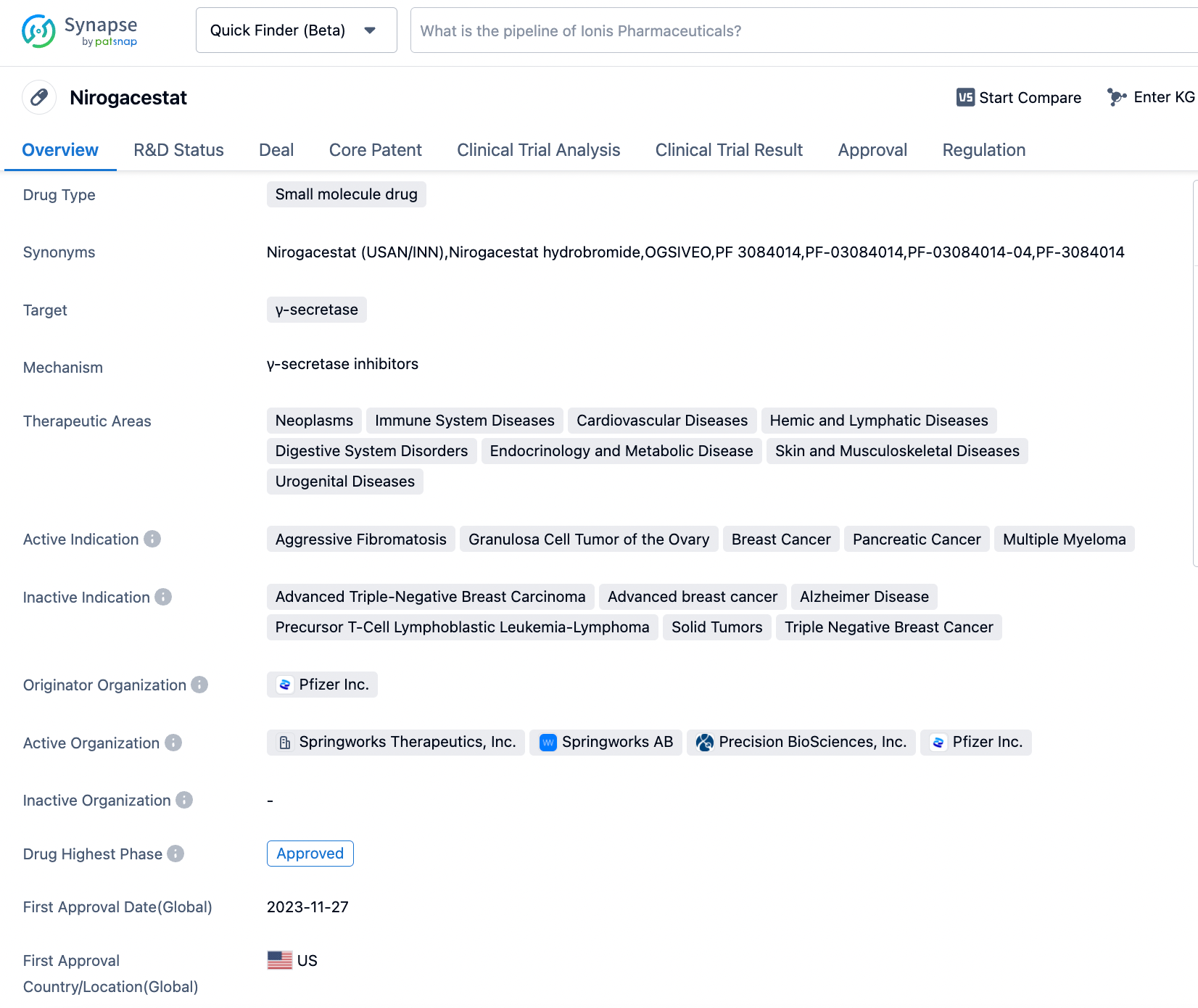
SpringWorks Therapeutics announced FDA approval of OGSIVEO™ (nirogacestat) as the first treatment for adult Desmoid tumor patients
SpringWorks Therapeutics, Inc., an enterprise operating in the biopharmaceutical sector with its efforts concentrated on tackling serious rare disorders and oncological conditions, has made public that the FDA has sanctioned the use of OGSIVEO™ (nirogacestat), a gamma secretase inhibitor administered orally, to manage progressive desmoid tumors in adults in need of systemic therapy. Prior to this authorization, the FDA had conferred several special status designations on nirogacestat, which included the breakthrough therapy designation, fast track status, and the orphan drug classification specifically for the management of desmoid tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Our group is privileged to be the pioneering provider of the first treatment sanctioned by the FDA for individuals diagnosed with desmoid tumors. "The patient population has long anticipated an efficacious therapy that can reduce the size of their tumors and offers a significant reduction in pain, which is frequently cited as the most incapacitating effect experienced by those afflicted with desmoid tumors," commented SpringWorks CEO Saqib Islam.
Islam expressed his satisfaction, noting, "The comprehensive labeling encompasses all adult patients experiencing tumor progression and notably mentions pain relief. We are optimistic that OGSIVEO will set a new paradigm in the treatment for those affected by these crippling growths."
"Desmoid tumors profoundly affect individuals and pose treatment challenges due to their propensity to aggressively invade surrounding tissues and the likelihood of reappearance after remission. OGSIVEO stands out as an advanced treatment with promising results demonstrated in shrinking these tumors and alleviating related symptoms," remarked Mrinal M. Gounder, M.D., an expert on sarcomas from Memorial Sloan Kettering Cancer Center.
Dr. Gounder elaborated, "From the physician's perspective, the positive outcomes from the DeFi study regarding OGSIVEO were inspiring, showcasing significant effectiveness against primary and crucial secondary measures, paired with an acceptable tolerance in terms of safety. This green light marks a significant milestone in treating this patient demographic."
Jeanne Whiting, the Executive Director Emeritus and Co-Founder of the Desmoid Tumor Research Foundation, expressed, "This milestone is the result of persistent efforts by patients, academic researchers, and the biopharmaceutical sector all working diligently to push the boundaries of scientific research."
Patients in the U.S. will be able to access OGSIVEO through a network of specialty pharmacies and distributors, with availability expected between five to ten business days. SpringWorks plans to submit an application to the European Medicines Agency for marketing authorization for OGSIVEO in desmoid tumor cases in the earlier part of 2024.
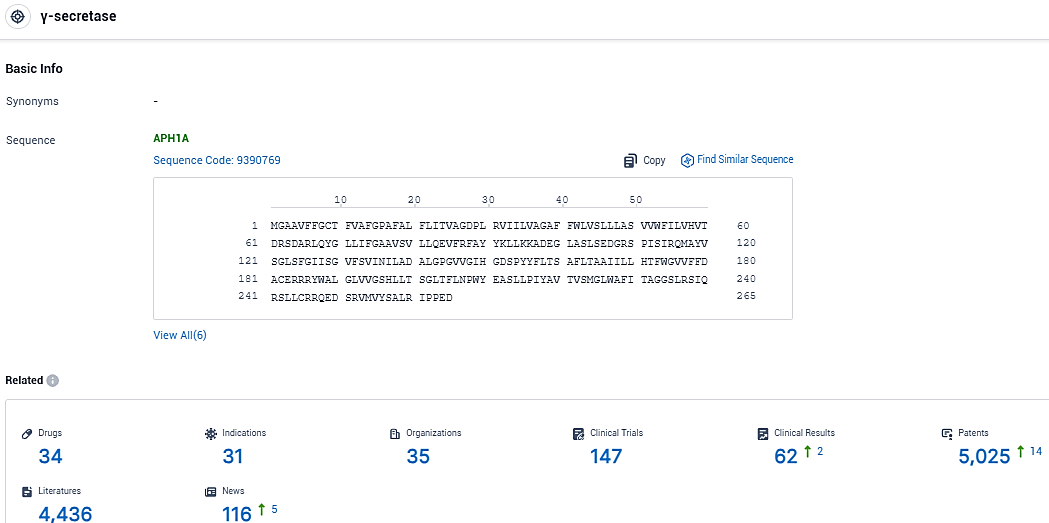
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 5, 2023, there are 34 investigational drugs for the γ-secretase target, including 31 indications, 35 R&D institutions involved, with related clinical trials reaching 147, and as many as 5025 patents.
OGSIVEO™ (nirogacestat) is an oral, selective, small molecule gamma secretase inhibitor approved in the United States for the treatment of adult patients with progressing desmoid tumors who require systemic treatment. OGSIVEO is not approved for the treatment of any other indication in the United States, or for any indication in any other jurisdiction by any other health authority.