TC BioPharm Gets Approval from FDA to Conduct Phase 1B IND for TCB-008 in Treating Acute Myeloid Leukemia
TC BioPharm PLC, a clinical-stage biotech firm that is working on developing a platform regarding allogeneic gamma-delta T cell treatments for cancer, has stated that the FDA has given the green light for the firm's application for a new drug under investigation intended for a Phase 1B trial in recurrent and refractory Acute Myeloid Leukemia.
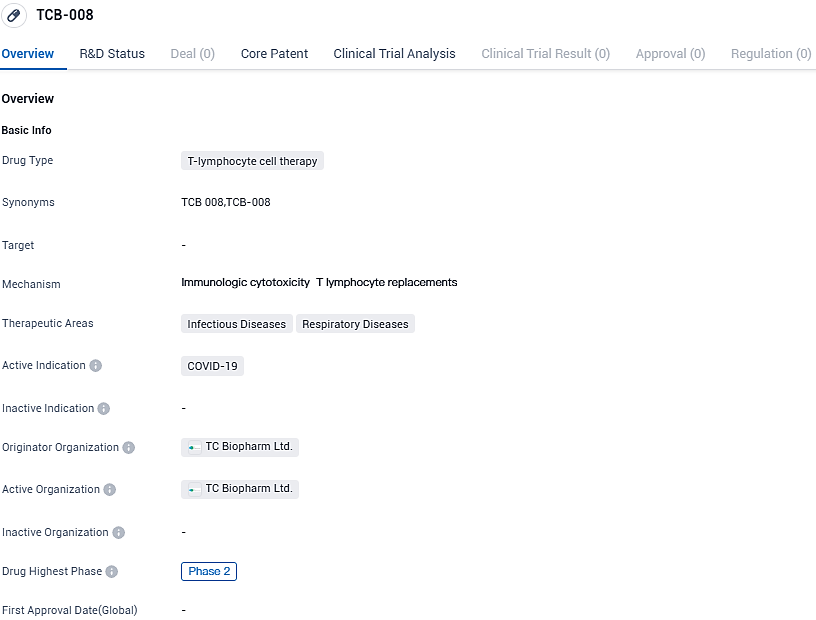
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The initial stage of investigation named ACHIEVE2, which is a Phase 1B research, will involve testing 9 participants in an escalating dose study. The study's aim is to measure safety levels and optimise the dosage. This Open-label research, which will take place in multiple centres in two parts, will assess the safety, persistence/expansion, and initial efficiency of both single and multiple IV doses of TCB008 in patients diagnosed with AML or MDS/AML. It permits patients to receive subsequent infusions of TCB008 up to three times following the first infusion, as decided by the investigator or their designate provided that the predetermined protocol criteria are met.
The accepting of the TCB-008 IND illustrates a considerable mark of achievement for TC BioPharm, marking the successful execution of our strategy that was shared with our shareholders in April," stated Bryan Kobel, TC BioPharm's Chief Executive. TC BioPharm is focusing its efforts on developing an improved treatment approach for the millions globally affected by blood and bone marrow cancers. Our goal is to make TCB008 the standard cure for these patients."
Furthermore, the firm intends to proceed with the ACHIEVE trial in AML in the UK. They anticipate submitting proposed changes to the protocol by the end of the year to ensure alignment with the dosing and other requirements of the ACHIEVE2 trial for TCBP's major product.
Kobel added, "By 2024, we anticipate numerous milestones and turning points, both in our US trials and our continuing ACHIEVE studies, along with the expansion of our platform. Since the announcement of our revised strategy this year, TC BioPharm continues to successfully reach key milestones. We aim to maintain this productive trajectory in 2024 and we are thankful for our shareholders' faith in our vision."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
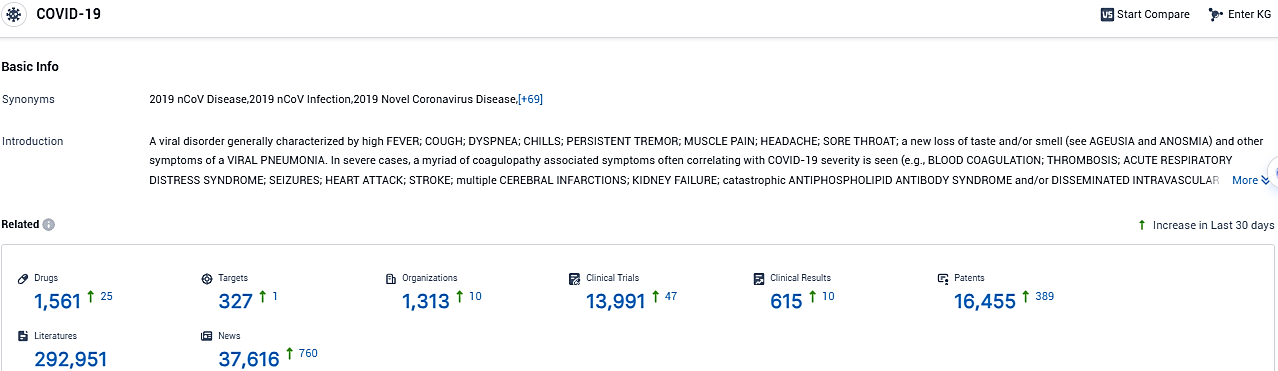
According to the data provided by the Synapse Database, As of November 30, 2023, there are 1561 investigational drugs for COVID-19, including 327 targets, 1313 R&D institutions involved, with related clinical trials reaching 13991, and as many as 16455 patents.
TCB-008 is a T-lymphocyte cell therapy drug being developed by TC Biopharm Ltd. for the treatment of COVID-19. It has reached Phase 2 of clinical development, suggesting potential efficacy in combating the disease. Further research and testing will be necessary to determine the drug's overall effectiveness and safety profile.