Tectonic Therapeutics Begins Phase I Trial of TX45 for Group II Pulmonary Hypertension in Patients with HFpEF
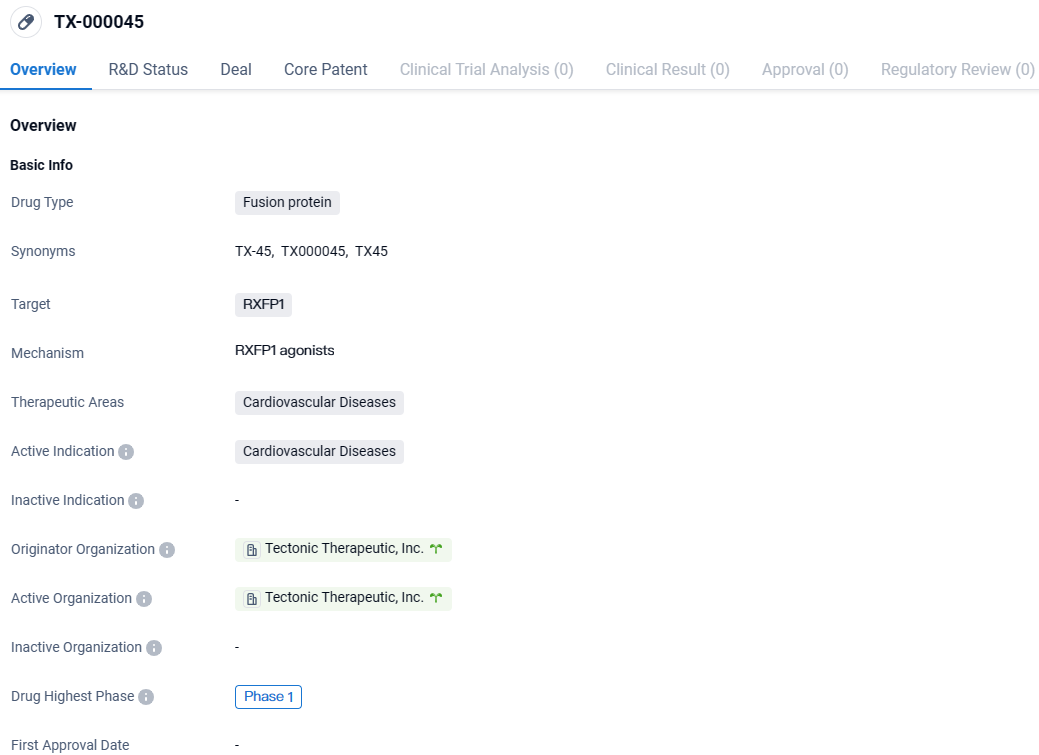
Tectonic Therapeutic, Inc., a non-public biotechnology firm focusing on the development of proteins aimed at GPCRs for therapeutic use, initiated by co-founders Timothy A. Springer and Andrew C. Kruse affiliated with Harvard Medical School, has officially declared the commencement of participant recruitment for their Phase 1B clinical trial. This trial is designed to evaluate the investigational molecule TX45, which is a fusion of relaxin with an Fc segment intended to function as a long-duration agent.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
This research aims to assess the tolerability and cardiovascular responses of a one-time administration of TX45 to subjects suffering from Heart Failure with preserved Ejection Fraction-induced Group 2 Pulmonary Hypertension. In America, this subgroup of Group 2 PH arising from HFpEF affects upwards of 600,000 individuals and is linked to profound clinical deterioration and an elevated risk of death. Critically, the absence of sanctioned treatments for this medical issue remains.
Alise Reicin, M.D., who leads Tectonic as its President and Chief Executive Officer, remarked, "Our objective is to harness the beneficial effects of relaxin such as its capacity to dilate blood vessels, reduce fibrosis, and curb inflammation to enhance therapeutic outcomes for individuals diagnosed with Group 2 PH." She added, "The distinctive biomolecular profile of relaxin has been presumed to hold considerable promise in managing heart diseases. Through this investigation, we anticipate gathering essential pharmacological and cardiovascular data relating to TX45."
Despite the inherent challenge of establishing a long-lasting therapeutic effect with the natural relaxin protein due to its naturally rapid degradation, preliminary findings from Tectonic's active Phase 1A study indicate TX45's promise in surpassing these hurdles and in potentially claiming the title of a top-tier treatment. We foresee the announcement of initial Phase 1A findings around the middle of 2024, with further results from the subsequent Phase 1B study in Group 2 PH and HFpEF patients due in 2025. A randomized, controlled Phase 2 trial is envisioned to kick off during the latter half of 2024.
The Phase 1B research of TX45 with subjects having Group 2 PH associated with HFpEF involves a single administration study that is open-label in design. This study will investigate the safety profile, the capacity for patient acceptance, and immediate cardiovascular effects following intravenous administration of TX45. Researchers will monitor changes in cardiovascular measurements obtained via right heart catheterization and echocardiographic techniques.
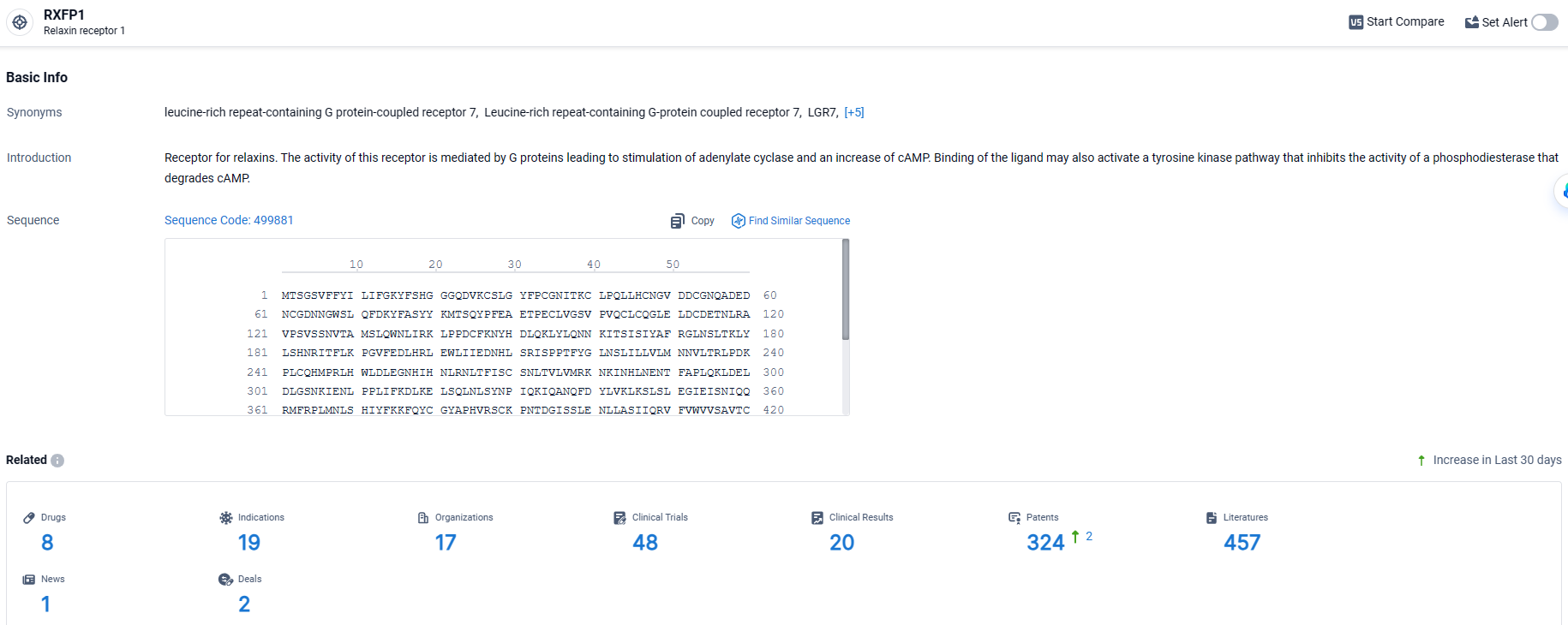
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 28, 2024, there are 8 investigational drugs for the RXFP1 target, including 19 indications, 17 R&D institutions involved, with related clinical trials reaching 48, and as many as 324 patents.
TX45 targets RXFP1 suggests that it may have a unique mechanism of action that is tailored to modulate the activity of this receptor. RXFP1 is known to play a role in cardiovascular diseases, making it a promising target for drug development in this therapeutic area. Further details regarding the drug's specific mechanism of action, clinical trial results, or potential benefits and risks are not available. Additional research and clinical trials will be necessary to determine the safety and efficacy of TX45 in treating cardiovascular diseases.