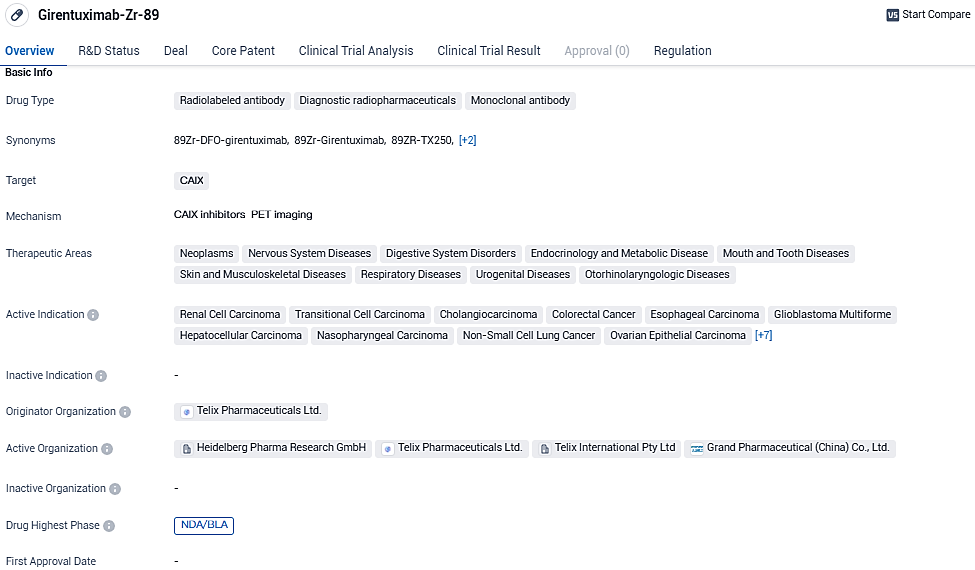
Telix has filed a licensing request for its biological product, TLX250-CDx, marketed as Zircaix™, aimed for use in renal cancer visualization
Telix Pharmaceuticals Limited has officially reported the submission of a Biologics License Application to the U.S. FDA for the experimental diagnostic tool TLX250-CDx (Zircaix™, 89Zr-DFO-girentuximab), which is utilized in PET scans specifically for detecting clear cell renal cell carcinoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Under the Breakthrough Therapy status, TLX250-CDx has been allowed a phased evaluation process, with the ability to submit and have sections of the application assessed progressively, according to a schedule established in coordination with the FDA. As part of its Biologics License Application (BLA), Telix is seeking Priority Review to hasten the review period if approved.
Telix Group's CEO and Managing Director, Dr. Christian Behrenbruch, commented, "Reaching this stage represents a significant stepping stone for Telix, setting the stage for TLX250-CDx to potentially become accessible for patient use in the United States by 2024, contingent upon the completion of the regulatory scrutiny and subsequent endorsement."
"Should the FDA endorse TLX250-CDx, it will become the inaugural targeted radiopharmaceutical diagnostic agent for renal cancer offered to U.S. patients. The FDA's collaborative stance throughout the Breakthrough Therapy process has been instrumental as we endeavor to introduce this pioneering, noninvasive, unique 89Zr-labeled monoclonal antibody imaging agent into the healthcare marketplace," added James Stonecypher, Chief Development Officer at Telix.
Associate Professor Brian Shuch, MD, who heads the Kidney Cancer Program and holds the Alvin & Carrie Meinhardt Endowed Chair in Kidney Cancer Research at the UCLA Institute of Urologic Oncology, mentioned, "The ZIRCON trial showcased the heightened sensitivity and specificity of this sophisticated diagnostic imaging tool. Upon authorization, it will become the exclusive agent that zeroes in on carbonic anhydrase IX, a key marker in renal carcinoma. It meets a considerable gap, ensuring greater reliability in the diagnosis of ccRCC, which stands as the most prevalent and virulent type of renal cancer."
Telix's application is anchored in the success of its worldwide Phase III ZIRCON study, which hit its primary and secondary objectives as announced in November 2022. Telix has initiated an expanded access scheme in the U.S. and a named patient program in Europe, facilitating access to TLX250-CDx outside clinical trials for those patients who lack comparable or satisfactory alternative treatment options.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
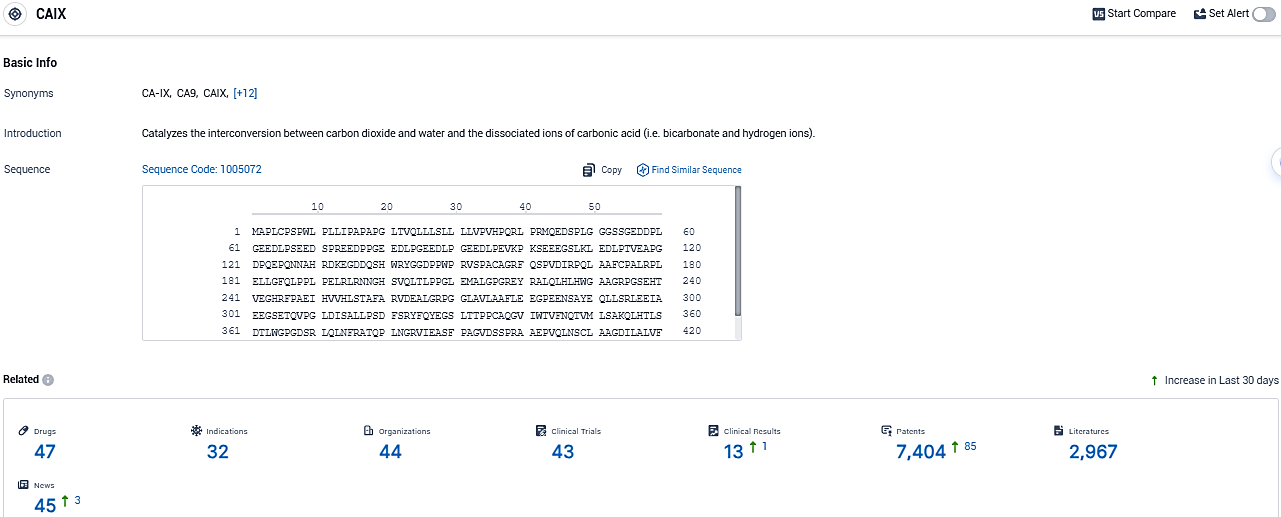
According to the data provided by the Synapse Database, As of December 27, 2023, there are 47 investigational drugs for the CAIX target, including 32 indications, 44 R&D institutions involved, with related clinical trials reaching 43, and as many as 7404 patents.
TLX250-CDx targets CAIXand has shown promise in the treatment of various cancers and other diseases. With its highest phase of development being NDA/BLA globally and Phase 1 in China, the drug has been classified as a breakthrough therapy, highlighting its potential to address unmet medical needs in the field of biomedicine.