Telomir Pharmaceuticals Announces Promising Telomir-1 Anti-Aging Drug Results in D.C. Press Event
Telomir Pharmaceuticals, Inc., which is operating at a pre-clinical stage and concentrates on researching Telomir-1 for the treatment of conditions associated with aging, recently summarized its presentation of significant pre-clinical findings about Telomir-1. This occurred during a prominent event that took place at the National Press Club in Washington, D.C., on April 15, 2024.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Leading the presentation, Dr. Chris Chapman, co-founder, chairman, CEO, and chief medical officer of Telomir, alongside Dr. Michael Roizen, an age reversal advisor at the company, highlighted the impressive potential of Telomir-1 to enable self-reparation of the body and reduce age-related deteriorations.
In an effort to maintain transparent communication about their discoveries, Drs. Chapman and Roizen shared critical findings at the event. They revealed the impacts of Telomir-1, a pioneering molecule aimed at regulating telomerase, and extending telomeres. Evidence was provided showing an enhancement of telomerase activity by 40% and the extension of telomeres in human cell studies conducted in vitro.
Furthermore, the presentation covered significant results from preclinical studies in animals, identifying benchmarks such as improvement or decline in gait, evaluation of joint health, dynamic capacity to bear weight, along with analyses in clinical chemistry, blood studies, telomere conditions, and synovial fluid modifications.
Dr. Chapman, during their speech at the National Press Club, talked about the unveiling of Telomir-1 as a strategy against aging ailments on a national platform. He mentioned, “The preliminary research findings are promising and show Telomir-1's capacity to address ailments linked with old age. We are dedicated to keeping the public informed as we proceed through the stages of pre-clinical testing, Investigational New Drug, and Advanced Investigational New Drug (AIND) applications, and further.”
Dr. Roizen, who also is the Chief Wellness Officer Emeritus at the Cleveland Clinic, stressed the innovative methods of Telomir-1 in addressing conditions related to aging such as osteoarthritis, underlining the essential science involving the control of stem cells.
Dr. Roizen praised the crucial support from Telomir-1’s expert panel on longevity, set up in December 2023. This panel, consisting of 14 experts from diverse domains like medicine, medical ethics, public relations, law, and economics, showcases a comprehensive strategy to directing the research agenda for Telomir-1.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
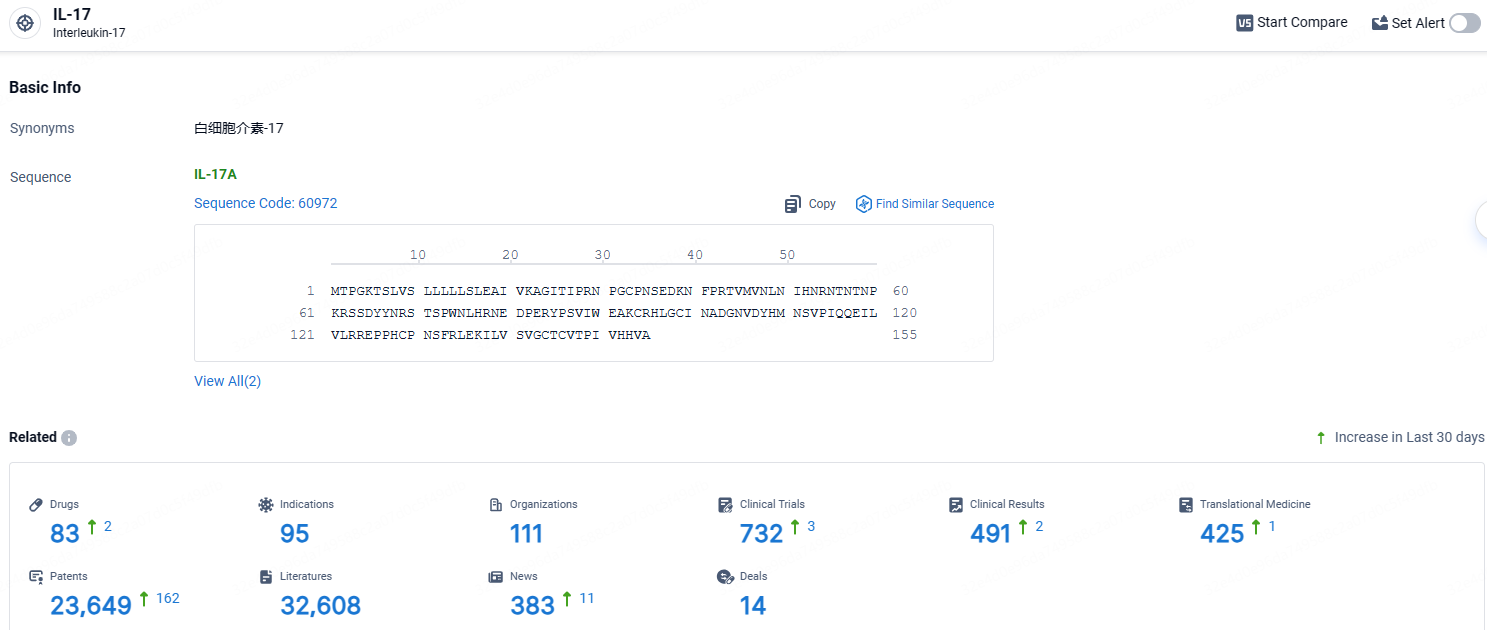
According to the data provided by the Synapse Database, As of April 18, 2024, there are 83 investigational drugs for the IL-17 target, including 95 indications, 111 R&D institutions involved, with related clinical trials reaching 732, and as many as 425 patents.
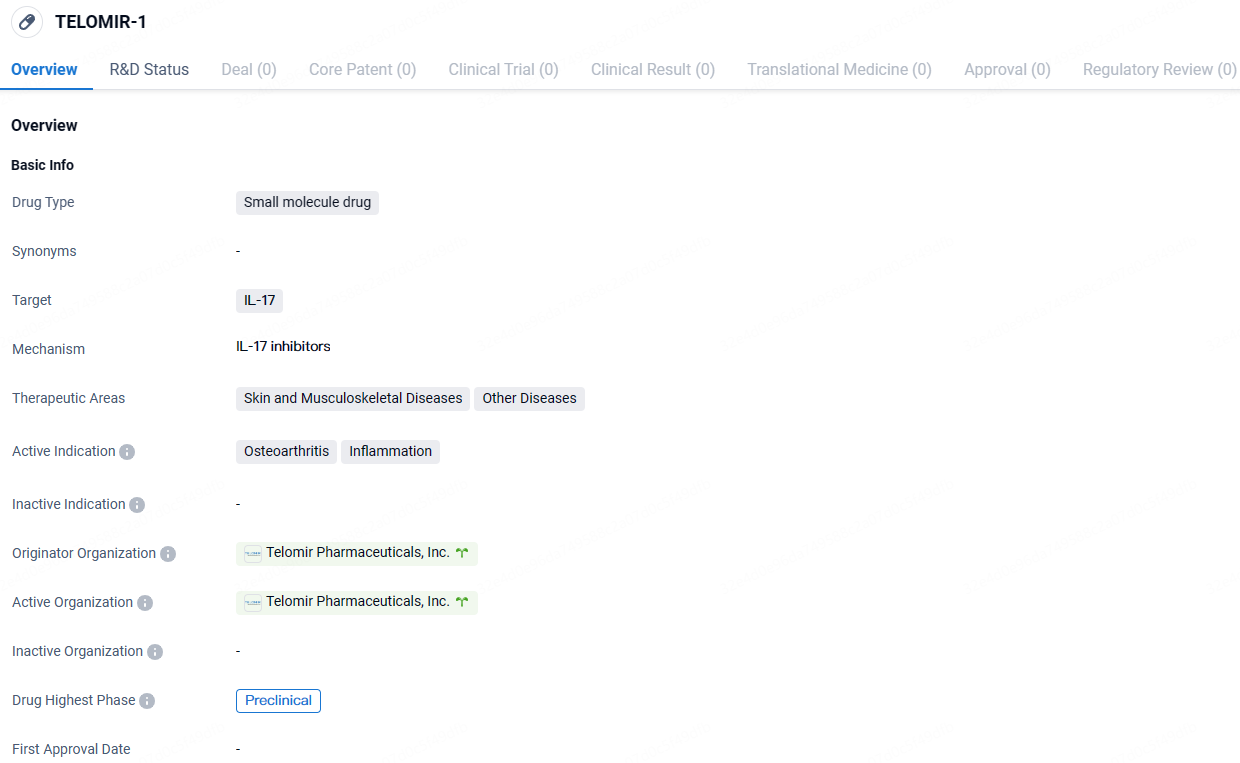
TELOMIR-1 targets IL-17 and is being developed for the treatment of Osteoarthritis and Inflammation. Currently in the preclinical phase, TELOMIR-1 is still undergoing laboratory testing and has not yet progressed to human clinical trials. Further research and development will be necessary to determine the safety and efficacy of this drug in treating the indicated conditions.