The FDA approved updated packaging for Kite Pharma's Yescarta® with survival rate information
Kite, part of Gilead Sciences, has divulged that the U.S. Food and Drug Administration sanctioned an amendment to the Yescarta® (axicabtagene ciloleucel) product labeling. This update integrates data from the pivotal Phase 3 ZUMA-7 trial, which presents conclusive evidence of enhanced overall survival (OS) when Yescarta is employed as an early second-line therapy aimed at curing individuals who have large B-cell lymphoma that has either returned or failed to respond after initially completing treatment within the preceding twelve months.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The recent modification to the Yescarta product information draws upon the conclusions of the ZUMA-7 trial, which reported a 27.4% decrease in mortality risk when compared to standard of care (SOC), equating to a 38% relative enhancement in overall survival (OS). Upon reviewing the median follow-up data of approximately 46.7 months, a significant statistical advancement in OS was observed in participants treated with Yescarta over those who underwent conventional treatment, even though a majority of the latter group eventually received cell therapy outside of the study protocol. At the 39-month mark, the rates of OS were measured at 55.9% for the Yescarta group in contrast to 46% in the group receiving SOC.
Frank Neumann, MD, PhD, Kite's Senior Vice President and Global Head of Clinical Development, stated, “The latest update to Yescarta's U.S. labeling is a crucial move to bolster the conviction of medical professionals in choosing Yescarta as an immediate treatment for patients with large B-cell lymphoma after initial disease progression or relapse.”
Frank Neumann further commented, "The data on overall survival from our ZUMA-7 trial attests that Yescarta can surpass standard therapies in prolonging patients’ lives when employed as a secondary treatment option. This, combined with our established quick and dependable production process, aims to extend the life expectancy of those living with the condition."
Traditionally, SOC for patients with this condition has been a sequential method that ideally culminates in a stem cell transplant. Initially, patients undergo chemoimmunotherapy, followed by high-dose chemotherapy, and if they are able and fit, they proceed to autologous stem cell transplantation. Notably, in past practices, fewer than 40% of patients completed the entire series of treatments leading to stem cell transplant. In stark contrast, during the ZUMA-7 trial, 94% of individuals allocated to Yescarta successfully underwent a single infusion of the therapy.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
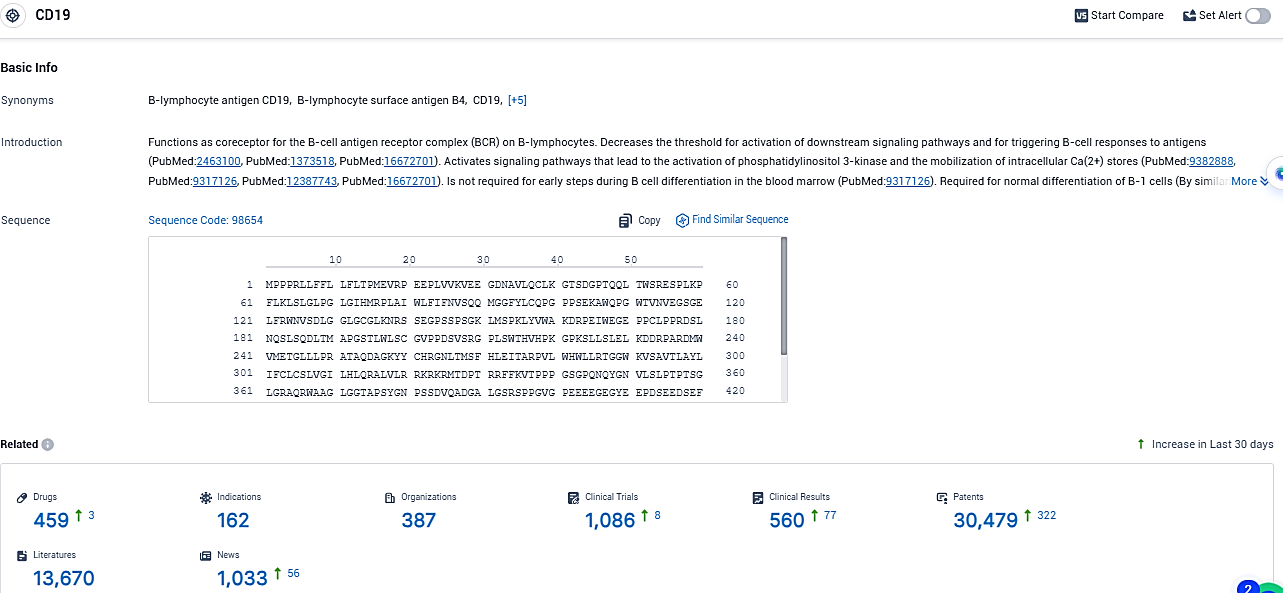
According to the data provided by the Synapse Database, As of December 29, 2023, there are 459 investigational drugs for the CD19 target, including 162 indications, 387 R&D institutions involved, with related clinical trials reaching 1086, and as many as 30479 patents.
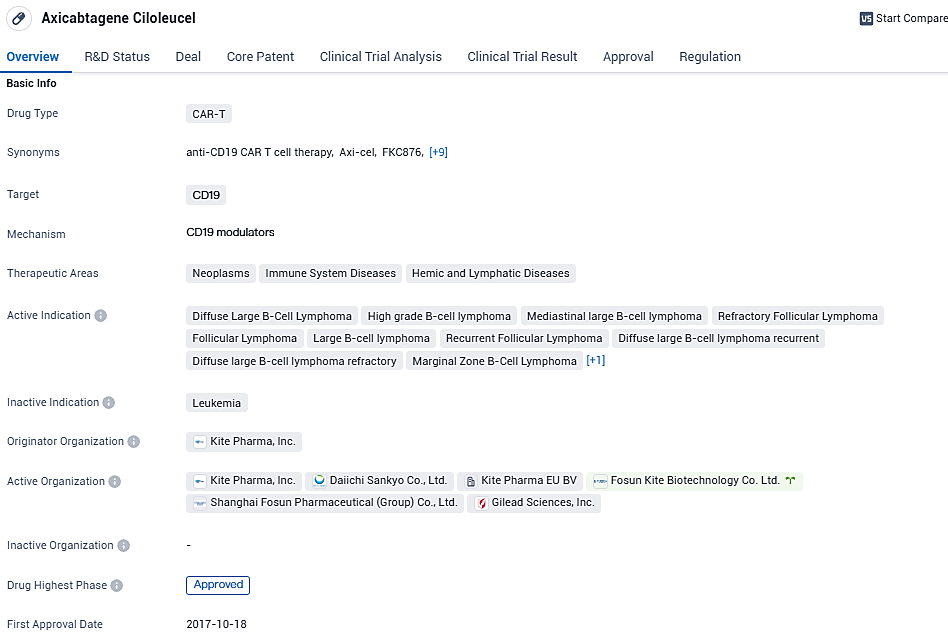
axicabtagene ciloleucel targets CD19 and has been approved for use in various therapeutic areas, particularly in the treatment of different types of lymphomas. With its first approval in the United States in 2017 and subsequent approval in China, axicabtagene ciloleucel has demonstrated its potential to address unmet medical needs. The regulatory designations it has received further highlight its importance in the field of biomedicine.