The phase 2 clinical trial results of MoonLake's innovative nanobody treatment, Sonelokimab, have shown positive outcomes, effectively alleviating psoriasis
MoonLake Immunotherapeutics recently announced positive results from the global Phase 2 clinical trial, ARGO, assessing the efficacy and safety of its nanobody, sonelokimab, in patients with active psoriatic arthritis (PsA). The trial met its primary endpoint and all key secondary endpoints, with significant improvements in the Psoriasis Area and Severity Index of the patients after 12 weeks of treatment with sonelokimab.
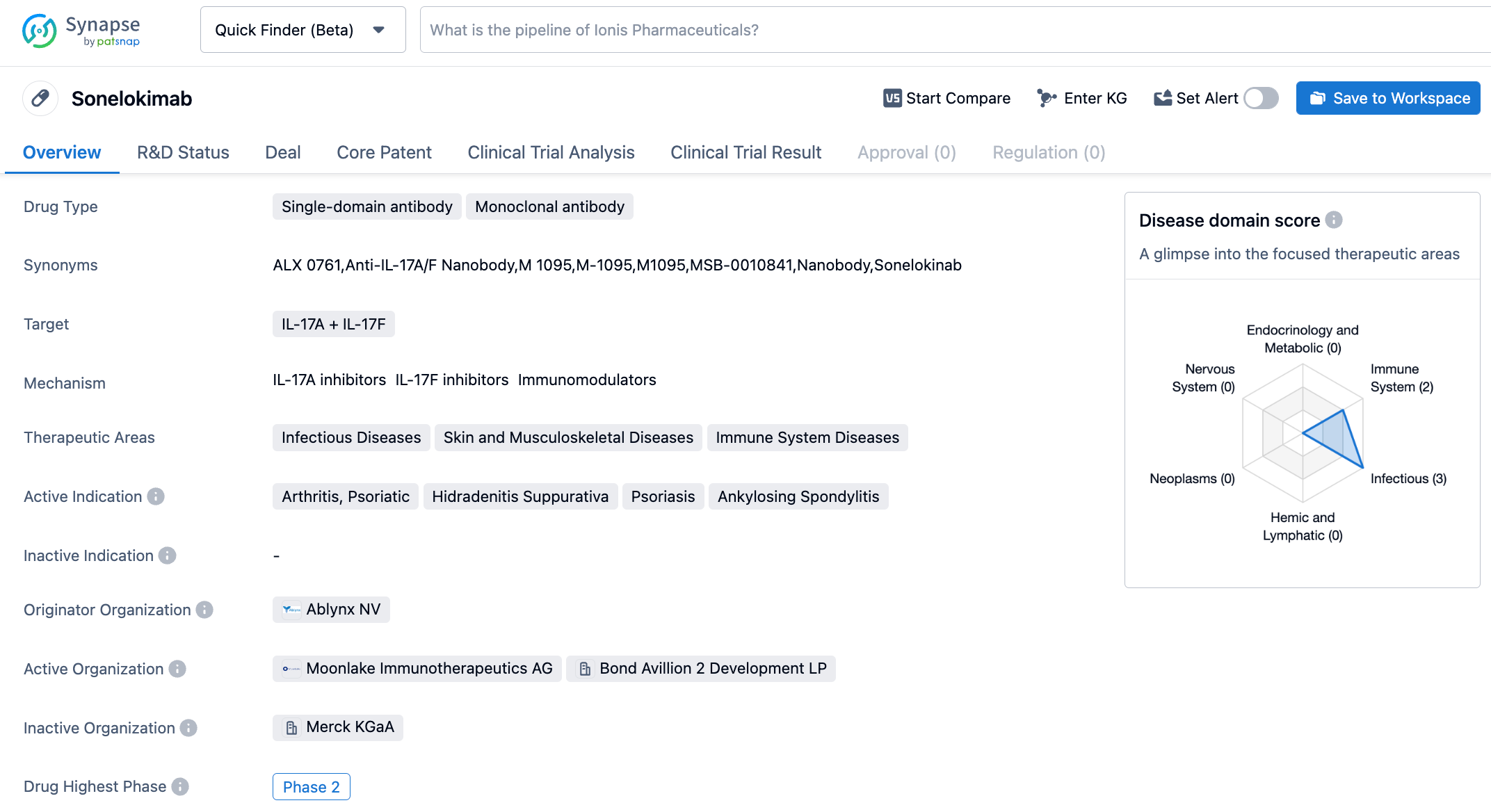
Sonelokimab is a new, innovative nanobody-based drug developed by MoonLake Immunotherapeutics, targeting IL-17A + IL-17F. It incorporates a nanobody that binds to serum albumin to extend its half-life and is composed of three VHH domains, with the third central domain binding to human serum albumin, which aids in further enrichment of sonelokimab at the inflammation edema sites. At present, clinical trials of sonelokimab are being conducted for psoriasis, hidradenitis suppurativa, and psoriatic arthritis.
In the ARGO trial, the primary endpoint was reached: the proportion of patients who achieved a 50% reduction in the American College of Rheumatology Disease Activity Score (ACR50) response at week 12 was significantly higher in patients treated with either 60 mg or 120 mg sonelokimab compared to those who received placebo. The drug discontinuation rate at week 12 was remarkably low (less than 4%) in the ARGO trial, similar to what was previously observed in trials of sonelokimab for psoriasis and hidradenitis suppurativa. Sonelokimab safety was consistent with previous study results, with no new safety signals observed. Specifically, the incidence of oral candidiasis in sonelokimab-treated patients was less than 2%, with no cases causing drug discontinuation. No cases of inflammatory bowel disease (IBD), major adverse cardiovascular events (MACE), or suicidal ideation and behavior (SI/B) were observed. Overall, sonelokimab continues to demonstrate robust safety, with no SI/B or liver enzyme elevation signals related to sonelokimab treatment identified so far in the entirety of the sonelokimab clinical program.
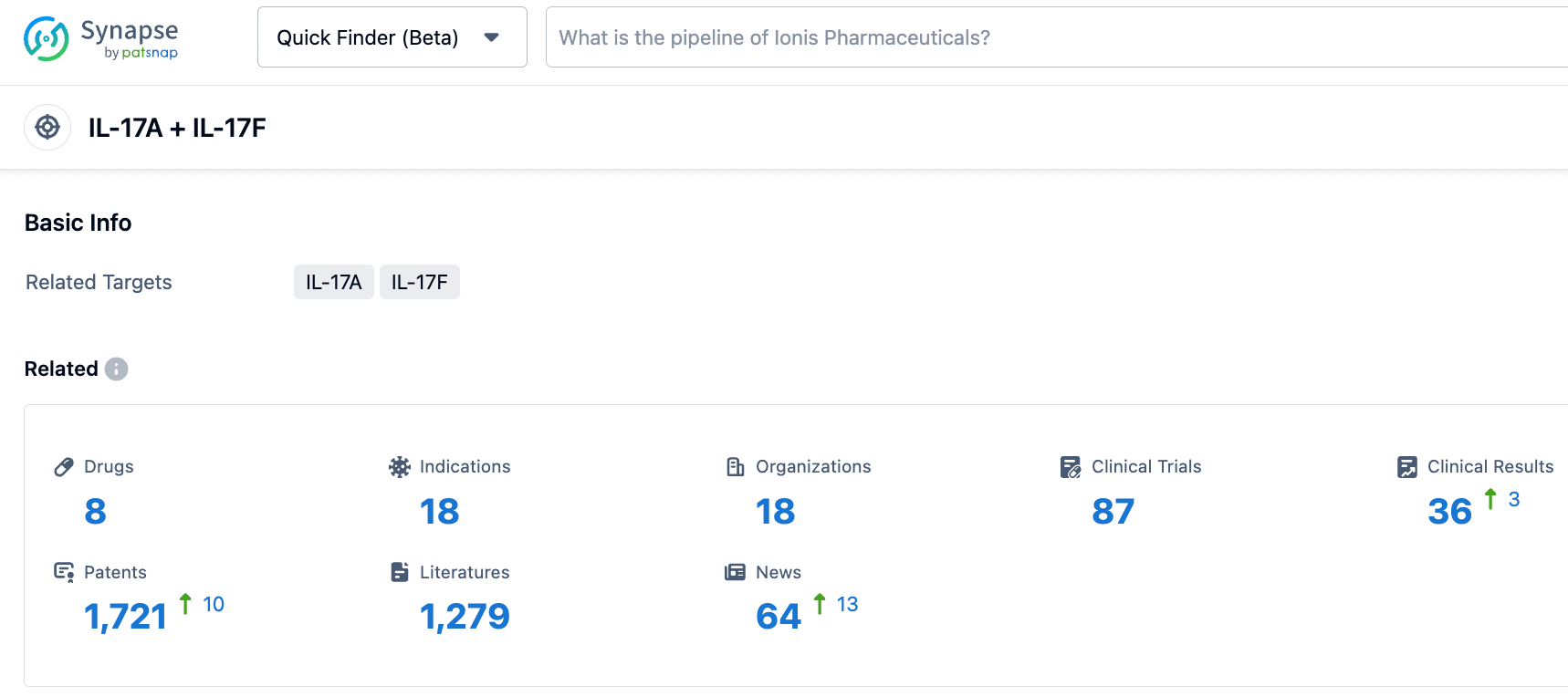
According to information released by the synapse database as of November 9, 2023, there are eight drugs in development targeting IL-17A + IL-17F, encompassing 18 indications, 18 research institutions, 87 related clinical trials, and as many as 1721 patents. Cosentyx, a groundbreaking product from Novartis and the world's first monoclonal antibody targeting IL-17A, has seen its global sales consistently rise since its market debut, reaching $4.8 billion in 2022, consolidating its flagship status. With its positive clinical trial data, sonelokimab has the potential to carve out a niche in the arena of autoimmune diseases.