The Phase 3 LUNA 3 trial of Rilzabrutinib successfully achieved its main goal in treating immune thrombocytopenia
The recent LUNA 3 phase 3 trial findings showed that administering 400 mg of rilzabrutinib orally twice daily effectively met the primary goal of sustaining platelet response in adults suffering from persistent or chronic immune thrombocytopenia. Rilzabrutinib's safety characteristics were in line with those observed in earlier research.
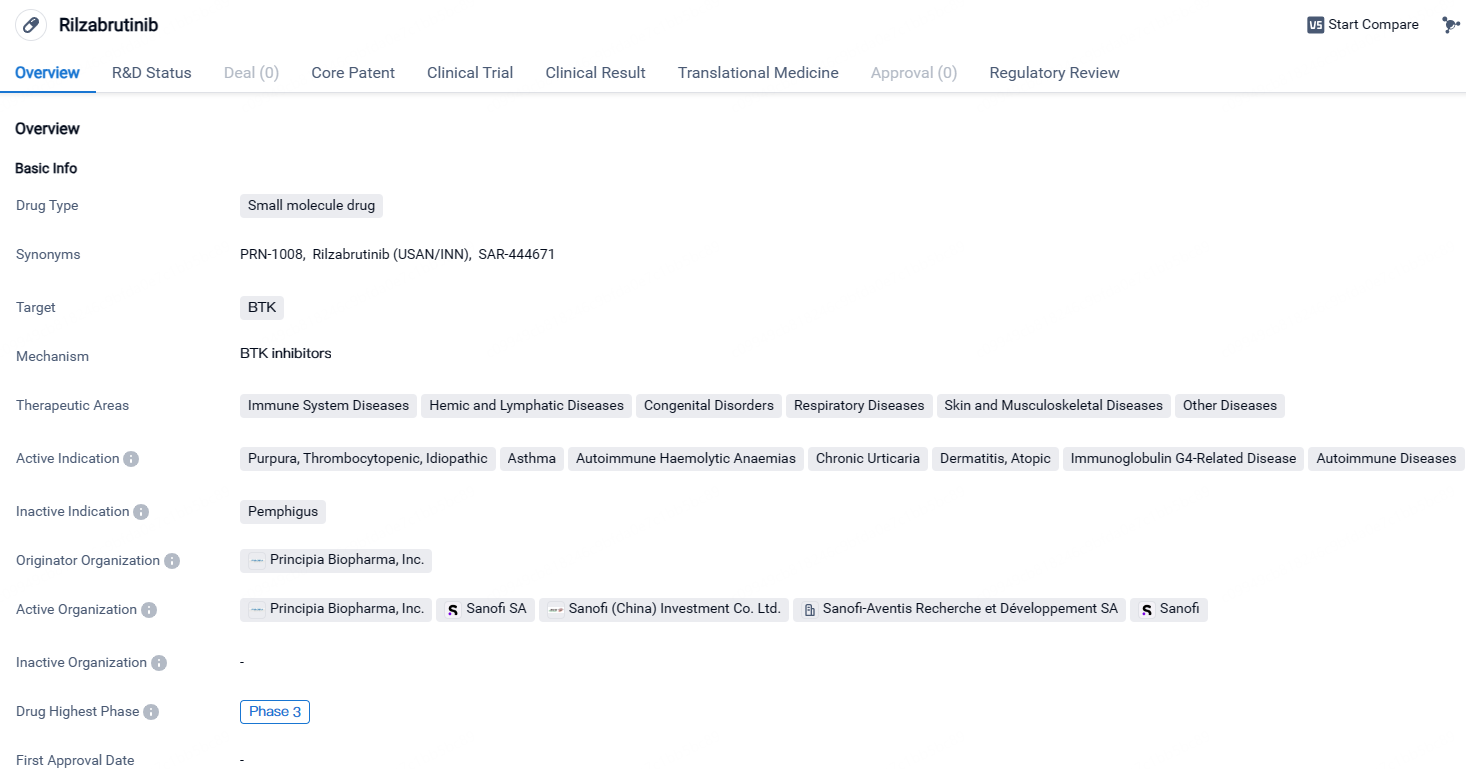
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The LUNA 3 trial successfully reached its primary objective, showing a significant percentage of individuals treated with rilzabrutinib had a sustained platelet response when compared to those given a placebo. This important outcome was observed in a group of patients with primary ITP who did not respond to previous treatments.
Participants in the study previously had an average of four different ITP therapies and entered the study with a median platelet count of 15,000/μL. Furthermore, significant improvements in critical secondary objectives further highlight the therapeutic promise of rilzabrutinib for those affected by long-standing and chronic ITP.
In November 2020, the US Food and Drug Administration assigned Fast Track Designation to rilzabrutinib for the management of ITP. Furthermore, the drug had already received Orphan Drug Designation.
Houman Ashrafian, the Executive Vice President, Head of Research and Development at Sanofi, remarked, “This study's findings bolster the vision of rilzabrutinib as a pioneering oral, reversible BTK inhibitor capable of offering meaningful clinical improvement to people affected by severe immune-related conditions such as ITP. These critical findings affirm our dedication and proficiency in addressing rare hematological conditions and our capacity to develop a new generation of tailored and selective small-molecule inhibitors, which show enhanced effectiveness and safety profiles when compared to existing treatments.”
ITP represents a severe, acquired autoimmune blood condition defined by the immune-driven destruction of platelets and reduced platelet production, leading to thrombocytopenia and elevated risks of severe bleeding.
Furthermore, ITP patients frequently suffer from notable declines in life quality, experiencing issues like fatigue and cognitive impairments. Through its dual action mechanism that both lowers the production of harmful autoantibodies and reduces macrophage-driven platelet destruction, rilzabrutinib has the potential to tackle the core causes of various ITP-related complications.
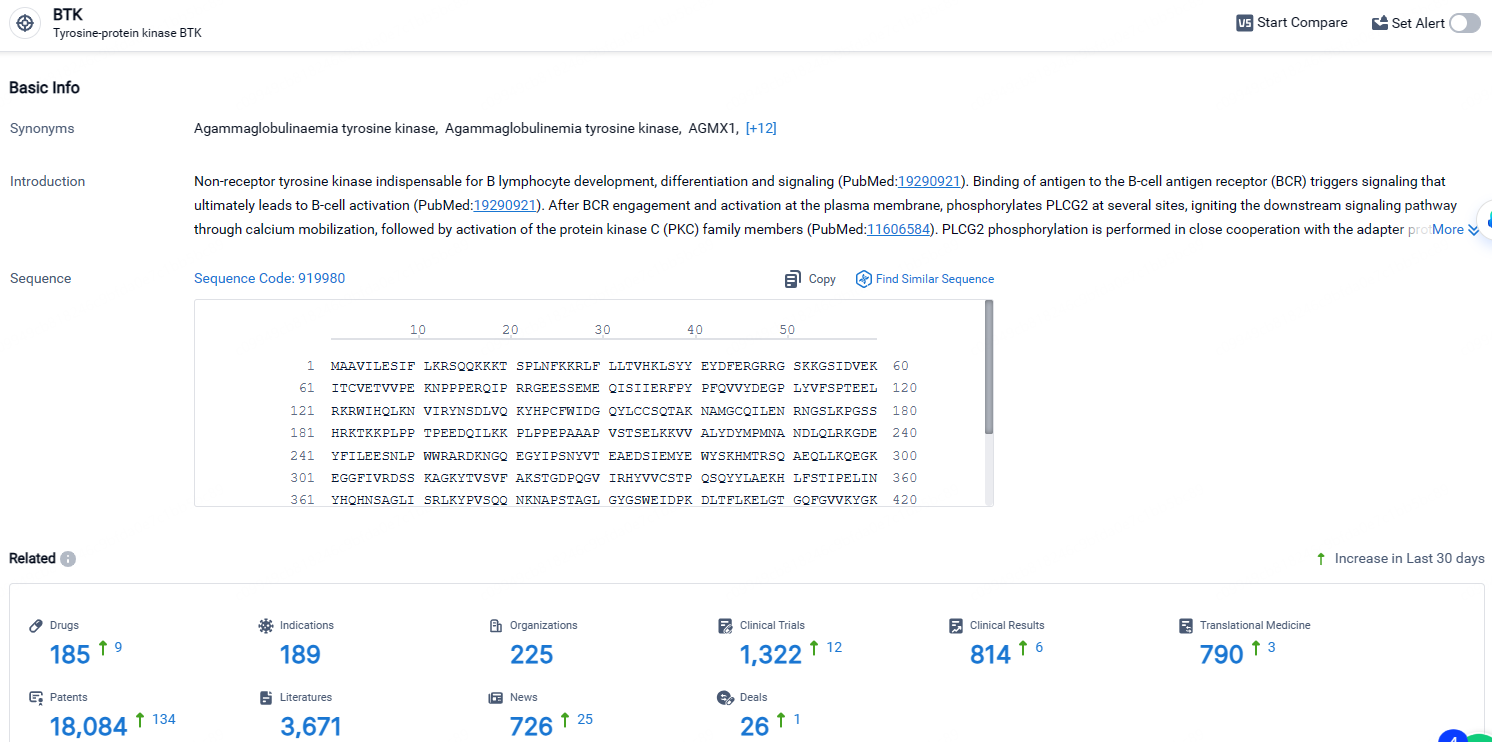
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 24, 2024, there are 185 investigational drugs for the BTK target, including 189 indications, 225 R&D institutions involved, with related clinical trials reaching 1322, and as many as 18084 patents.
Rilzabrutinib has reached Phase 3 in clinical development globally and in China. The drug shows potential in treating various immune-related disorders and has been designated as an orphan drug. Further research and clinical trials will be necessary to determine its safety and efficacy in treating the indicated conditions.