The Phase 3 TROPION-Breast01 trial showed Datopotamab Deruxtecan significantly improved progression-free survival in patients with HR positive, HER2 low/negative breast cancer
The TROPION-Breast01 phase 3 trial has returned positive results, showcasing a significant and clinically meaningful enhancement in the primary endpoint of PFS for patients treated with datopotamab deruxtecan. This improvement was statistically relevant in comparison to other chemotherapy options chosen by the investigator. This applies to those diagnosed with unoperable or metastatic hormone receptor positive, HER2 low or negative breast cancer who have undergone previous treatment with endocrine-based therapy and at least one other systemic therapy.
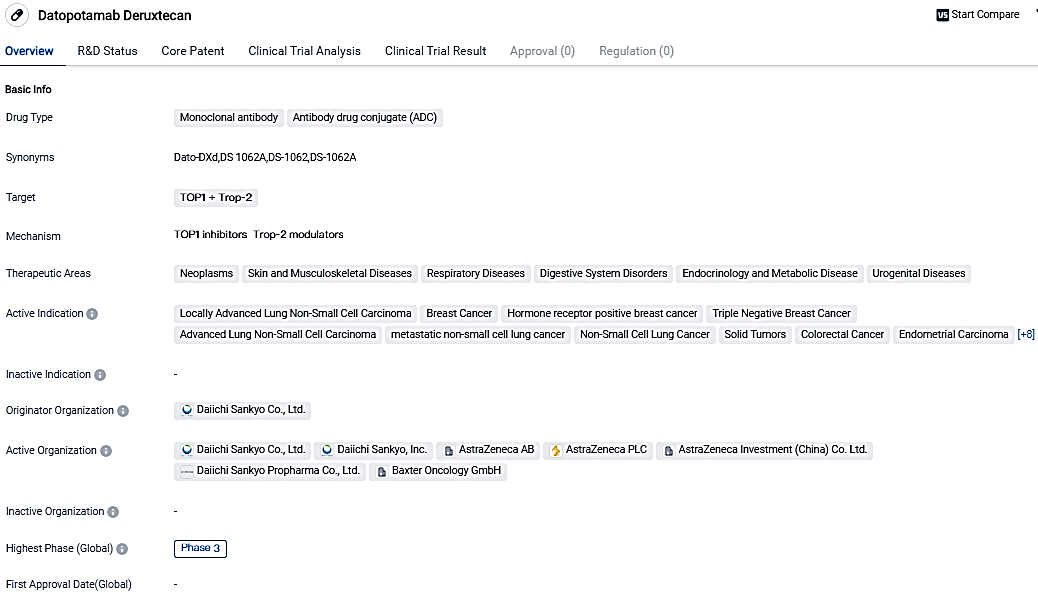
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Datopotamab deruxtecan, a TROP2 oriented DXd antibody drug mixture, is presently undergoing cooperative development by Daiichi Sankyo and AstraZeneca. The data regarding dual primary endpoint of overall survival were not finalized at latest interim examination and the research will proceed as projected to evaluate the OS.
The safety characteristics of datopotamab deruxtecan had no newly detected safety indications and were in line with prior clinical experiments with breast cancer. The rates for all grade interstitial lung disease were relatively low.
Annually, over two million individuals globally are identified with breast cancer. The most prevalent subtype is HR positive, HER2 low or negative breast cancer, which forms more than 65% of the confirmed cases. The introductory standard treatment for these patients typically is endocrine therapy, but most advanced disease patients tend to develop resistance, highlighting the importance of alternative options. TROP2 is a protein that is widely present in the HR positive, HER2 low or negative breast cancer.
Ken Takeshita, MD, Global Head, R&D, Daiichi Sankyo expressed his views about the promising primary results obtained from TROPION-Breast01 that could possibly make datopotamab deruxtecan an important option for treating patients with HR positive, HER2 low or negative breast cancer in the secondary metastatic context.
He added, "We are keen on leveraging the complete potential of this TROP2 oriented antibody drug combination across different breast cancer subtypes through our ongoing phase 3 program, which includes two trials focused on patients with triple negative breast cancer.”
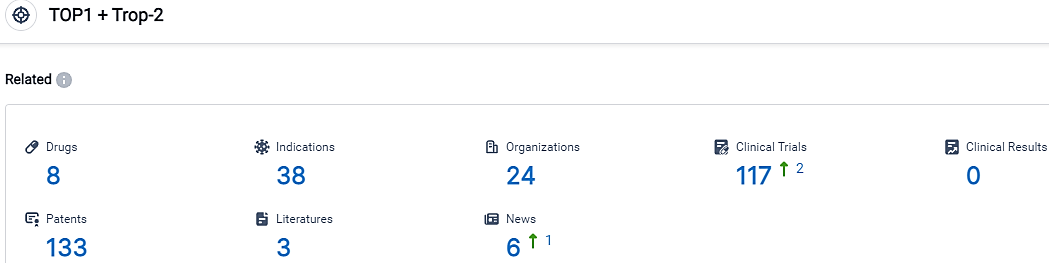
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 26, 2023, there are 8 investigational drugs for the TOP1 and Trop-2 target, including 38 indications,24 R&D institutions involved, with related clinical trials reaching 117,and as many as 133 patents.
Datopotamab Deruxtecan, a monoclonal antibody and ADC that targets TOP1 and Trop-2, is in the development process by Daiichi Sankyo Co., Ltd., and has advanced to Phase 3 trials worldwide and in China. Given its comprehensive therapeutic fields and active presentations in different types of cancer, Datopotamab Deruxtecan holds promise as a cutting-edge treatment solution in biomedicine.