Top 50 Pharmaceutical Companies R&D Progress: An Overview of Aurobindo's 22 Drug Pipelines
Aurobindo Pharma Ltd. is a pharmaceutical organization that was founded in 1986 and is based in Andhra Pradesh, India. The company operates in the field of biomedicine and has made significant contributions to the development of drugs in various therapeutic areas.
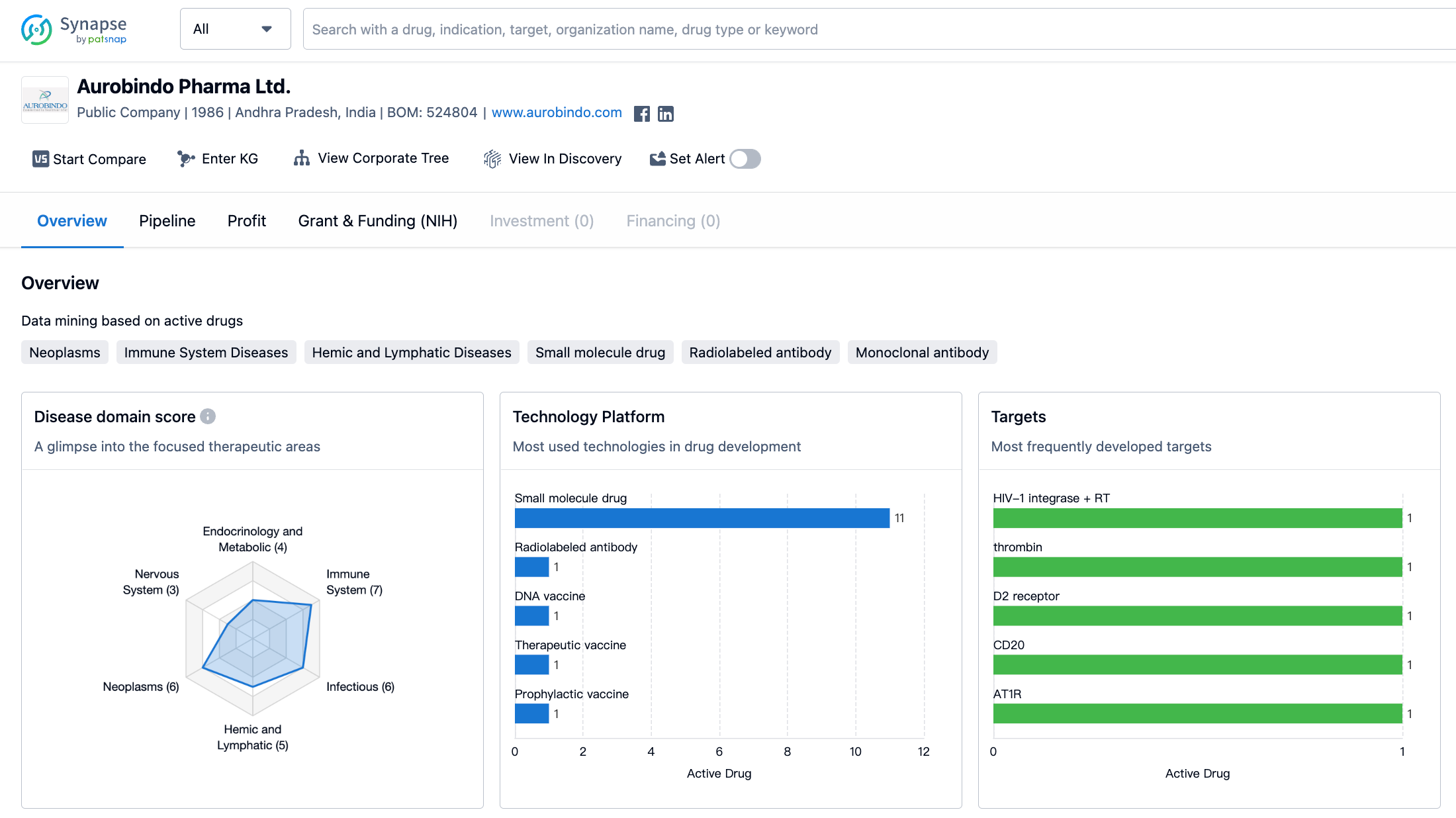
Aurobindo Pharma Ltd. has developed drugs in a wide range of therapeutic areas. The organization has the highest drug count in the field of immune system diseases, with a total of 7 drugs. This indicates that Aurobindo Pharma Ltd. has invested significant resources and efforts in developing treatments for diseases related to the immune system. Neoplasms, infectious diseases, and urogenital diseases are the next most focused therapeutic areas, with 6 drugs each. This suggests that the organization recognizes the importance of addressing these diseases and has dedicated resources to their research and development.
Cardiovascular diseases and hemic and lymphatic diseases are also areas of focus for Aurobindo Pharma Ltd., with 5 drugs each. This indicates the organization's commitment to developing treatments for conditions related to the cardiovascular system and blood disorders. Additionally, Aurobindo Pharma Ltd. has developed 4 drugs each for skin and musculoskeletal diseases, other diseases, and endocrinology and metabolic diseases. This demonstrates the organization's efforts to address a diverse range of medical conditions.
On the other hand, Aurobindo Pharma Ltd. has developed a relatively lower number of drugs for nervous system diseases, digestive system disorders, respiratory diseases, and eye diseases, with 3 drugs each. This suggests that the organization may have allocated fewer resources to these therapeutic areas compared to others.
The organization has developed drugs targeting various proteins and molecules involved in disease processes. These include HIV-1 integrase + RT, thrombin, D2 receptor, CD20, AT1R, NCC + SCNA, TYMS, HIV-1 RT + RT, HDAC, DHFR, and DNA. This indicates that Aurobindo Pharma Ltd. has a diverse portfolio of drug targets, which reflects its commitment to addressing different disease mechanisms.
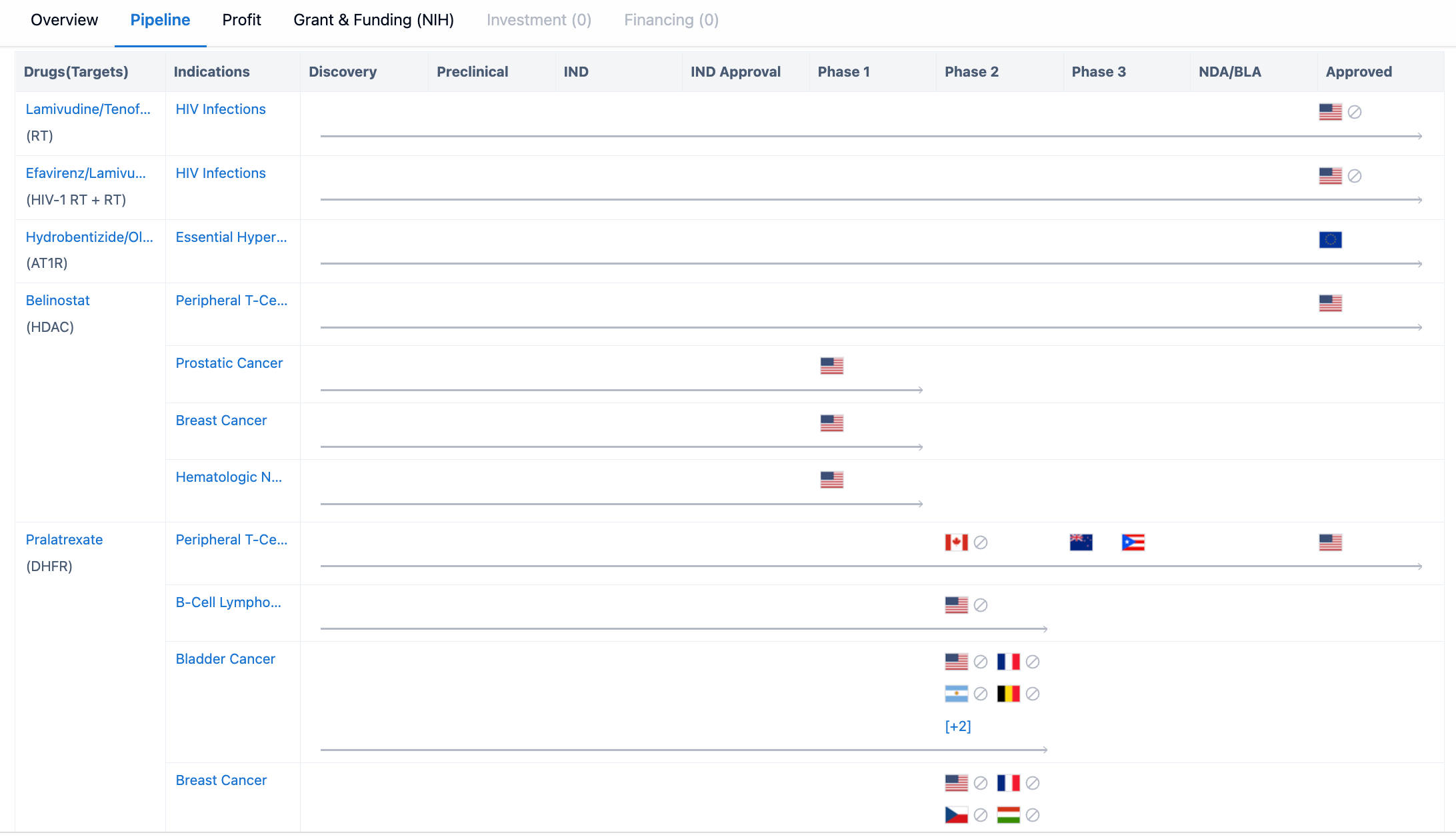
The organization has drugs at different stages of development, starting from the discovery phase. However, there are no drugs currently in the discovery or preclinical stages. This suggests that Aurobindo Pharma Ltd. may have recently shifted its focus towards more advanced stages of drug development, such as IND (Investigational New Drug) and IND Approval. The organization has 2 drugs in Phase 1, indicating that they have progressed beyond the initial stages of development. Additionally, there is 1 drug in Phase 2, which suggests that Aurobindo Pharma Ltd. is actively conducting clinical trials to evaluate the safety and efficacy of its drug candidates. However, there are no drugs currently in Phase 3, which is the final stage before seeking regulatory approval.
Aurobindo Pharma Ltd. has 1 drug in the NDA/BLA (New Drug Application/Biologics License Application) stage, indicating that the organization has submitted an application to regulatory authorities for approval. This suggests that Aurobindo Pharma Ltd. is actively seeking regulatory approval for at least one of its drug candidates. The organization has a significant number of drugs in the approved stage, with a total of 11 drugs. This indicates that Aurobindo Pharma Ltd. has successfully obtained regulatory approval for several of its drug candidates, allowing them to be marketed and sold to patients. Additionally, there are 7 drugs categorized as "Other" in the pipeline, which could include drugs in various stages of development or those with unique characteristics that do not fit into the traditional development phases.
In summary, Aurobindo Pharma Ltd. is a pharmaceutical organization that has made significant contributions to the development of drugs in various therapeutic areas. The organization has a diverse portfolio of drug targets and has focused its efforts on addressing diseases related to the immune system, neoplasms, infectious diseases, and urogenital diseases. Aurobindo Pharma Ltd. has drugs at different stages of development, with a significant number of drugs in the approved stage. This indicates the organization's commitment to bringing its drug candidates to market and providing treatments for patients. However, there is a need for further development in certain therapeutic areas, such as nervous system diseases, digestive system disorders, respiratory diseases, and eye diseases. Overall, Aurobindo Pharma Ltd. has established itself as a key player in the pharmaceutical industry and continues to contribute to the advancement of biomedicine.