TransCode Therapeutics Initiates First Phase Clinical Study
TransCode Therapeutics, Inc. (NASDAQ: RNAZ), an RNA oncology firm focused on enhancing cancer treatment through RNA-based therapeutics, has reported the start of its multicenter, open-label, Phase 1 clinical trial for its primary therapeutic candidate, TTX-MC138.
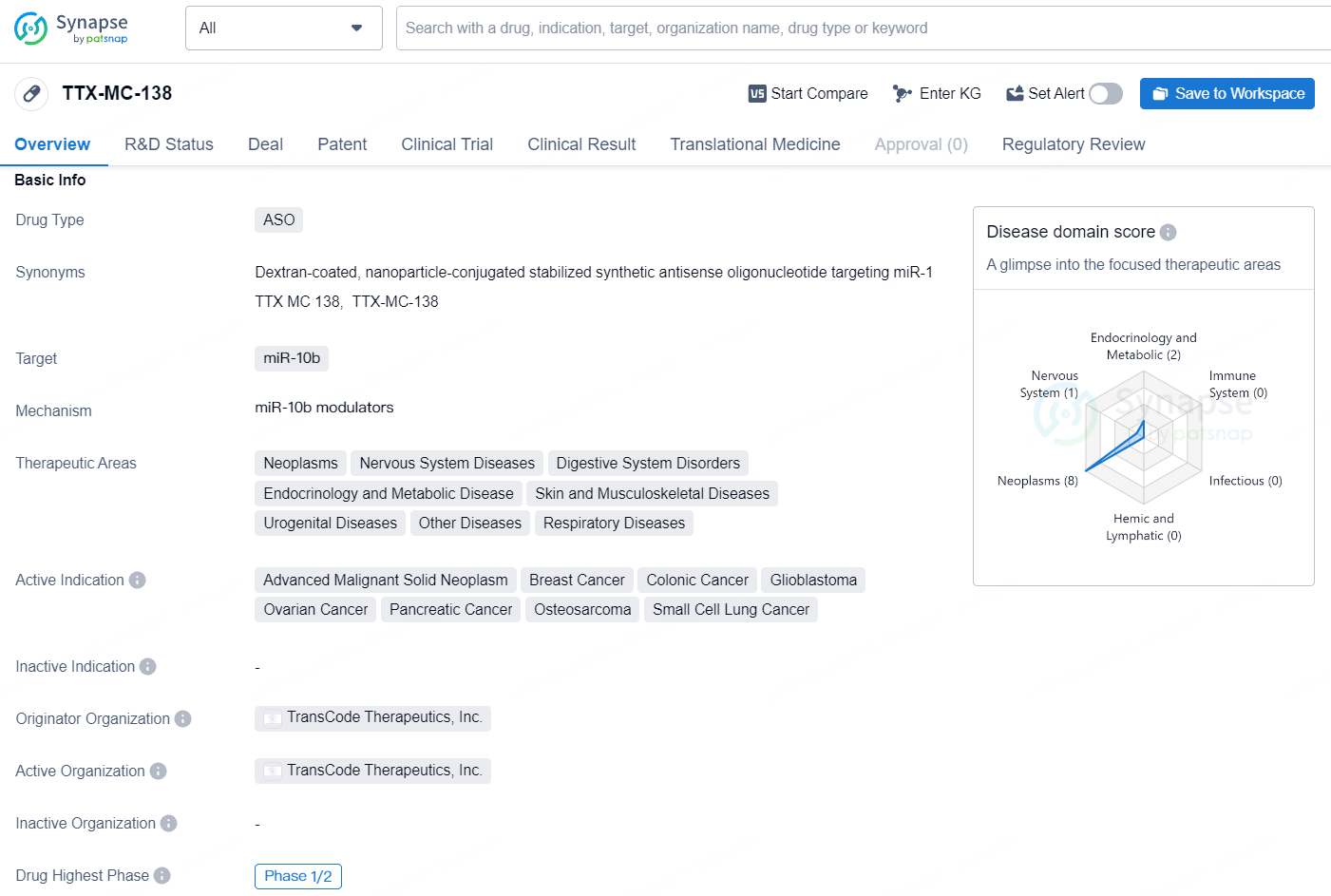
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Two clinical trial locations have been activated, with the expectation that patient enrollment will commence within the current quarter. TransCode might activate up to five clinical trial sites in total, with all sites anticipated to be activated this quarter.
The Phase 1 clinical trial aims to produce essential data to assess the safety of TTX-MC138 in patients with various metastatic solid tumors. It could also provide preliminary evidence of TTX-MC138’s clinical efficacy. The trial includes an initial dose-escalation phase, followed by a dose-expansion phase. The main goal of the dose-escalation phase is to determine the safety and tolerability of increasing doses of TTX-MC138. In the dose-expansion phase, the safety, tolerability, and anti-tumor efficacy of TTX-MC138 will be further examined in certain tumor types selected based on early results from the dose-escalation phase.
"We are excited to have attracted such significant interest in TTX-MC138 from leading oncologists at some of the nation’s most esteemed clinical trial sites," stated Sue Duggan, Senior Vice President, Operations, at TransCode. "Pushing forward breakthrough RNA therapeutics in clinical settings is our primary mission as a biotechnology company on the verge of pioneering technology," added Duggan.
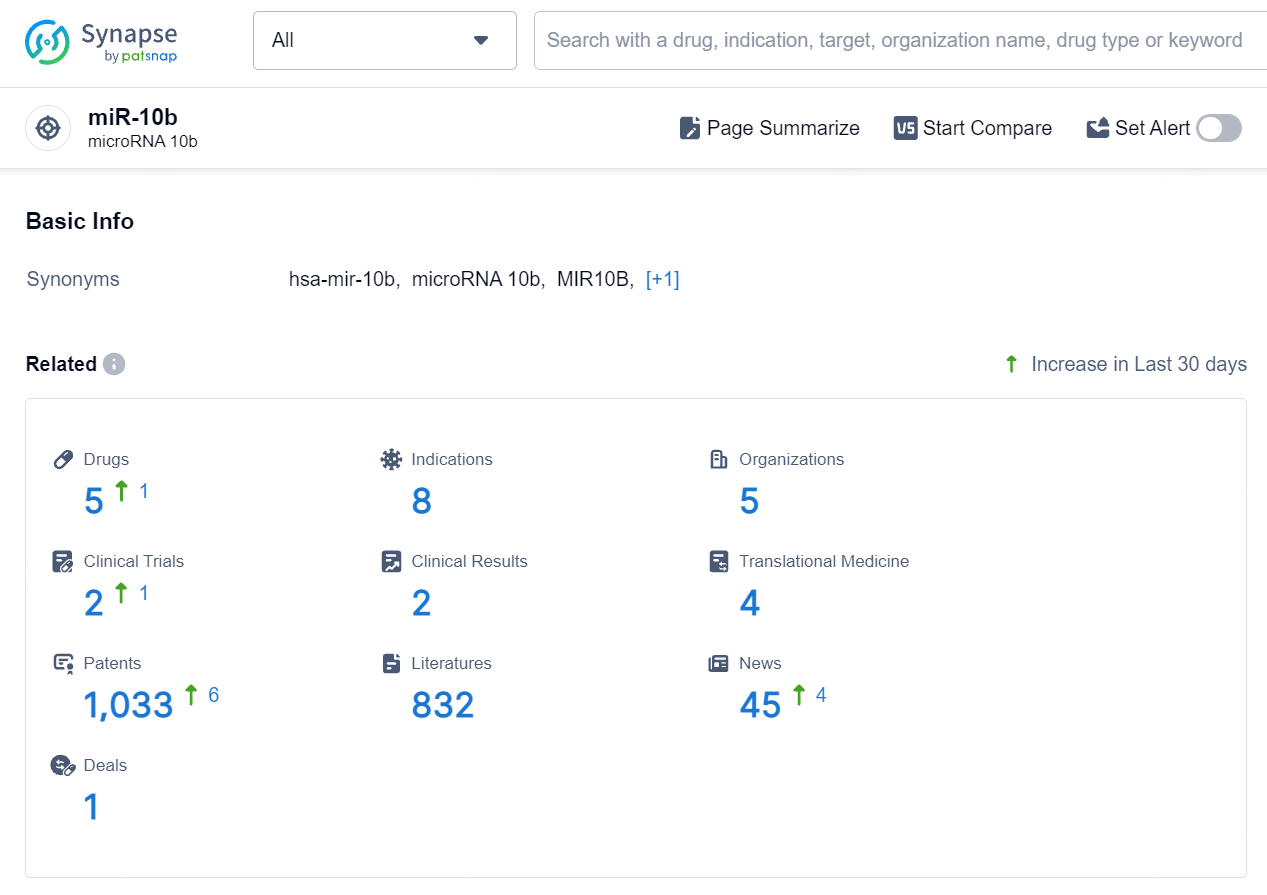
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 19, 2024, there are 5 investigational drugs for the miR-10b target, including 8 indications, 5 R&D institutions involved, with related clinical trials reaching 2, and as many as 1033 patents.
TTX-MC-138 is a drug classified as an antisense oligonucleotide (ASO) and it targets miR-10b. It is being developed to address a range of therapeutic areas, including neoplasms, nervous system diseases, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, urogenital diseases, other diseases, and respiratory diseases. The active indications for TTX-MC-138 include advanced malignant solid neoplasm, breast cancer, colonic cancer, glioblastoma, ovarian cancer, pancreatic cancer, osteosarcoma, and small cell lung cancer.