TScan Therapeutics Begins Phase 1 Trial of TCR-T Therapy in Solid Tumors
TScan Therapeutics, Inc., an entity engaged in clinical-stage biopharmaceutical developments, is dedicated to pioneering T cell receptor (TCR)-engineered T cell treatments aimed at cancer patients. The company revealed that the initial patient has received their dose in a Phase 1 study, which assesses the efficacy of TCR-T therapy in addressing solid tumors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
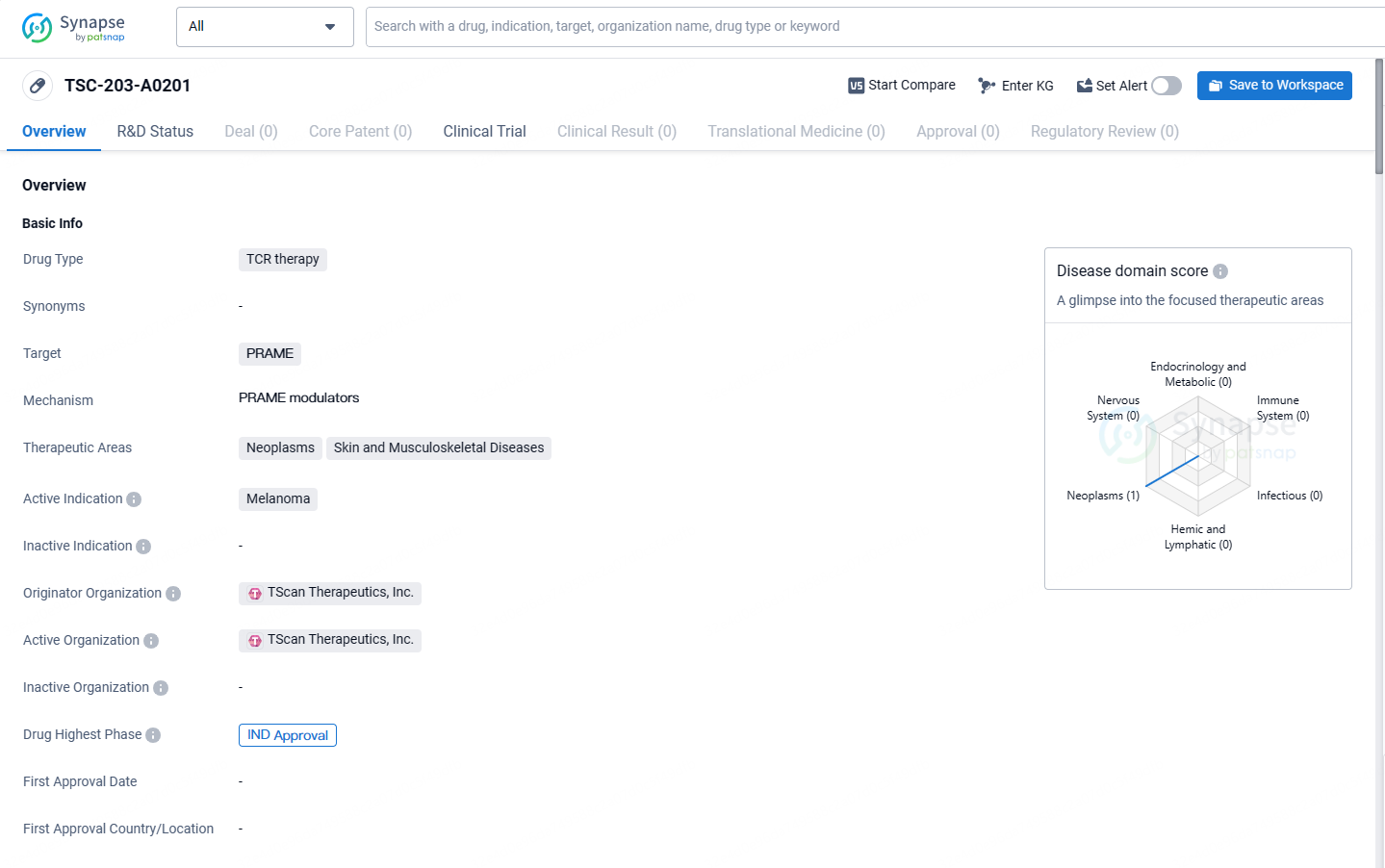
A patient with metastatic melanoma received treatment with TSC-203-A0201, which targets the cancer-associated antigen PRAME (PReferentially expressed Antigen in MElanoma).
"The initiation of treatment for the first patient in our solid tumor program marks a significant achievement as it aligns with the progress of our principal clinical initiatives," remarked Gavin MacBeath, Ph.D., the company's CEO.
“The enthusiasm surrounding this clinical trial remains high, with ongoing production of TCR-T therapies for an additional three patients. In this program, we have managed to get six TCR-Ts approved via existing IND submissions for patient treatment, and we are preparing to submit more INDs to significantly enhance the diversity of targets and HLA types within our ImmunoBank, thereby expanding the application of multiplex TCR-T therapy in treating solid tumors,” further stated Gavin MacBeath.
The Phase 1 clinical trial by TScan intends to evaluate the safety and practicability of T-Plex, an autologous customized TCR-T therapy. This therapy targets multiple peptide/human leukocyte antigens in patients demonstrating antigen-positive, locally advanced, unresectable, or metastatic solid tumors. The aim is to address tumor heterogeneity and the loss of HLA heterozygosity, which are prevalent resistance factors in solid tumors.
First-generation TCR-T therapies, which focus on single antigens, have demonstrated promising results in patients, although the duration of these responses commonly remains limited.
TScan’s strategy involves treating patients with multiple TCR-T products targeting differing cancer-associated antigens, anticipating this method will enhance responses and prolong their duration. Patients meeting the criteria are included in the clinical study treatment protocol, receiving one or multiple investigational TCR-T products tailored to the specific antigens present in their tumors.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
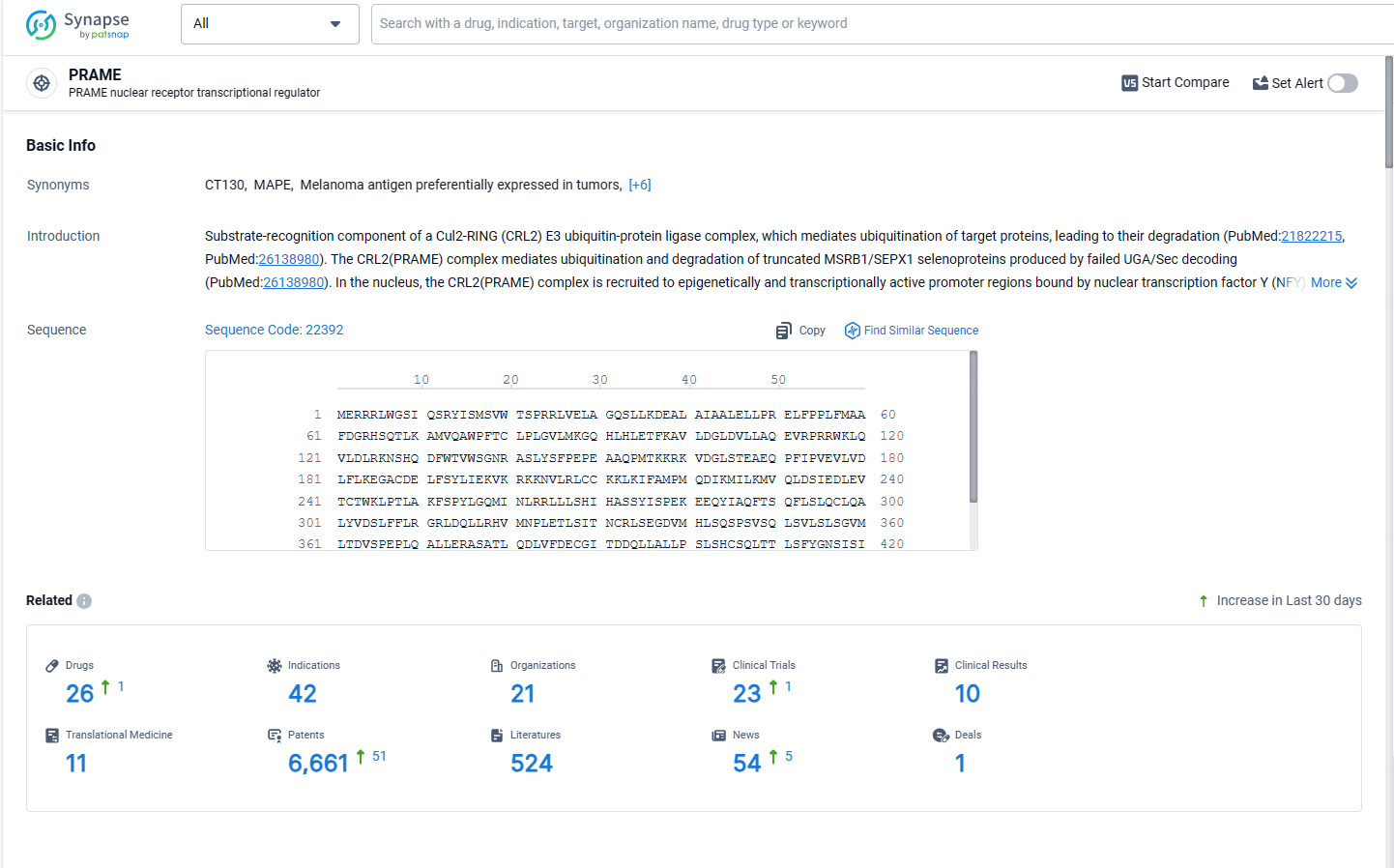
According to the data provided by the Synapse Database, As of May 13, 2024, there are 26 investigational drugs for the PRAME targets, including 42 indications, 21 R&D institutions involved, with related clinical trials reaching 23, and as many as 6661 patents.
TSC-203-A0201 targets the PRAME protein and is being developed for the treatment of melanoma. The drug has reached the IND approval stage, indicating its potential as a novel therapeutic option for patients with this aggressive form of skin cancer.