Unveiling the Shadows of Dolutegravir Treatment in the Youth: Neuropsychiatric Events and Suicidality Warning
In 2021, an estimated 2.7 million children and adolescents globally were living with HIV. Mental health issues including suicidal ideation (24% lifetime prevalence) and attempts (13%) are common among this group, likely due to multiple factors such as HIV itself, stigma, and antiretroviral therapy side effects. As dolutegravir is being rolled out globally as the preferred first-line HIV treatment, data on its potential neuropsychiatric side effects in youth are limited.

The ODYSSEY trial
A recent secondary analysis of the ODYSSEY trial, published in The Lancet Child & Adolescent Health, has shed light on the neuropsychiatric manifestations and sleep disturbances associated with dolutegravir-based antiretroviral therapy compared to standard-of-care treatment in children and adolescents living with HIV. The study, led by researchers from various institutions worldwide, highlights the importance of monitoring and screening for potential neuropsychiatric adverse events in young patients receiving dolutegravir.
Dolutegravir, a widely used antiretroviral drug, has shown excellent efficacy in treating HIV in adults. However, concerns have been raised about its potential neuropsychiatric side effects, which prompted the investigation into its impact on younger populations. The study aimed to evaluate the occurrence of neuropsychiatric adverse events and sleep problems in children and adolescents receiving dolutegravir-based treatment compared to alternative antiretroviral therapies.
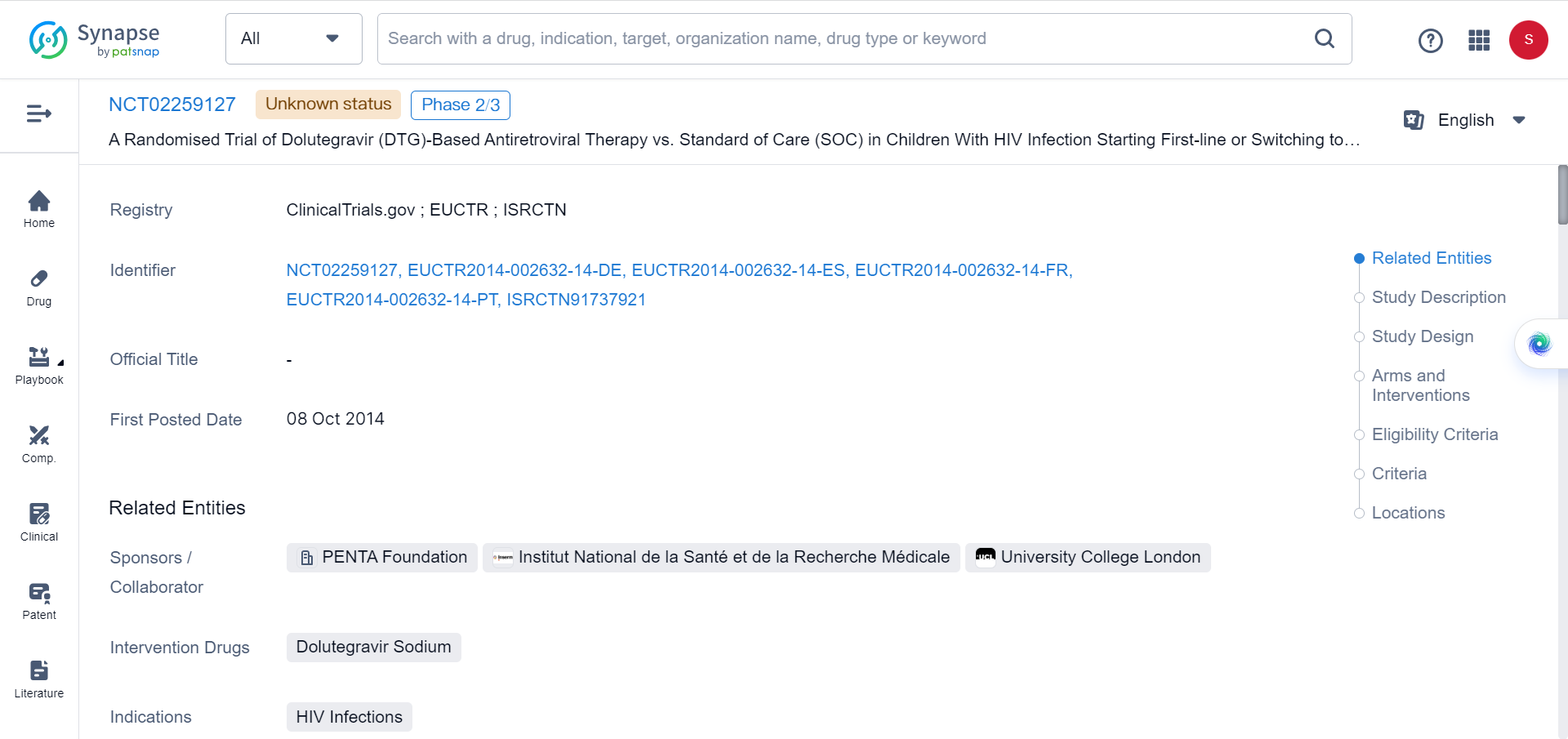
The ODYSSEY trial, an open-label, multicentre, randomised non-inferiority trial, enrolled 707 participants between September 2016 and June 2018. Among the participants, 50% were randomly assigned to dolutegravir-based antiretroviral therapy, while the remaining 50% received non-dolutegravir-based standard-of-care treatment. The trial included both patients initiating first-line and second-line antiretroviral therapy.
The study aimed to compare the effectiveness, safety, and tolerability of dolutegravir-based treatment against the standard-of-care treatment in pediatric and adolescent populations. Participants were monitored for a period of up to 96 weeks.
First- and second-line treatment regimens were designed according to ART resistance tests and treatment history. Dolutegravir doses were periodically adjusted in response to pharmacokinetic data throughout the trial. Additional drugs included in both study groups were administered in accordance with national guidelines.
Adverse events were systematically recorded through clinician reports. Clinicians meticulously documented all adverse incidents and distinguished severe or serious occurrences. An independent committee conducted a blinded review of all reported events.
Neuropsychiatric questionnaires were incorporated after the first amendment, around 10–12 months into the study. These paper questionnaires, designed to evaluate mood, sleep patterns, and issues, were regularly administered to participants aged 6 and above, as well as their caregivers. The questionnaires prioritized a select set of key questions to avoid overwhelming the respondents.
The adverse events
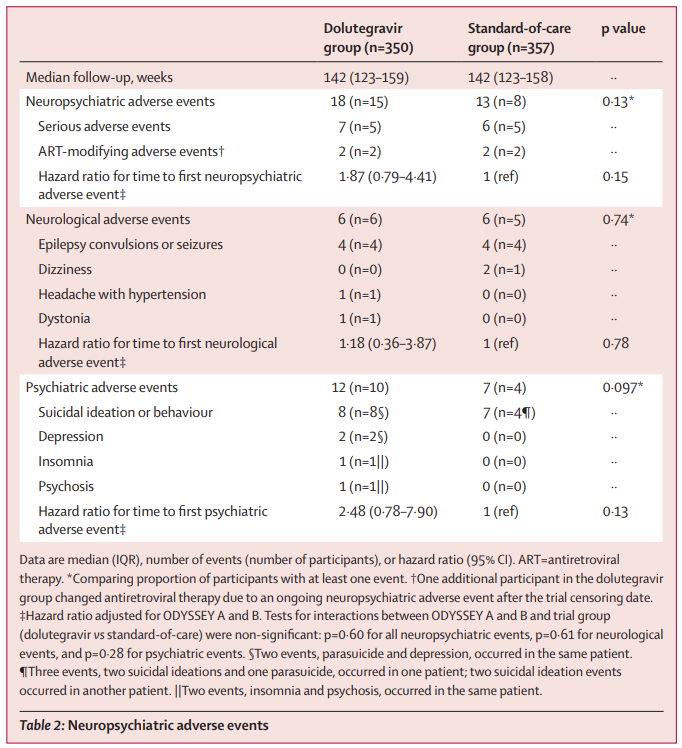
During the median follow-up period of 142 weeks, 31 neuropsychiatric adverse events were reported by 23 participants. Among these events, 15 were reported in the dolutegravir group, while eight were noted in the standard-of-care group. Though the difference in the proportion of participants reporting at least one adverse event was not statistically significant (p=0.13), the dolutegravir group had a higher number of participants reporting psychiatric events and suicidal ideation or behavior compared to the standard-of-care group.

In particular, eight individuals in the dolutegravir group reported symptoms of self-harm, as opposed to just one individual in the standard-of-care group (p=0.025). Moreover, a greater number of participants in the dolutegravir group reported feelings of life being not worth living (17 vs. five; p=0.0091) and suicidal thoughts (13 vs. none; p=0.0006) during one or more follow-up visits. It should be noted that the majority of these reports were transient, and the open-label nature of the study necessitates a careful interpretation of the results.
The study's findings suggest a low overall incidence of neuropsychiatric adverse events. However, they also highlight the need for healthcare providers and policymakers to consider implementing suicidality screening for children and adolescents undergoing dolutegravir-based treatment. The authors emphasize the crucial necessity of closely monitoring and addressing potential mental health issues within this susceptible demographic.
This research was sponsored by the Penta Foundation, ViiV Healthcare, and the UK Medical Research Council. As further investigations and discussions unfold, the findings of this study will likely contribute to a deeper understanding of the possible neuropsychiatric implications of dolutegravir-based antiretroviral therapy in children and adolescents. The overarching aim is to optimize the treatment and well-being of young individuals living with HIV.
Reference
A. Turkova et al., Neuropsychiatric manifestations and sleep disturbances with dolutegravir-based antiretroviral therapy versus standard of care in children and adolescents: a secondary analysis of the ODYSSEY trial, The Lancet Child & Adolescent Health, 2023 S2352-4642(23)00164-5.