U.S. Approves ENHERTU® for HER2 Positive Metastatic Solid Cancers Post-Initial Treatment
The U.S. Food and Drug Administration has granted authorization for ENHERTU (fam-trastuzumab deruxtecan-nxki), a collaborative cancer medication developed by Daiichi Sankyo and AstraZeneca. This approval is specifically for adult patients suffering from inoperable or metastatic solid tumors characterized by HER2 positivity, who have previously undergone systemic therapy and for whom there are no suitable alternative therapies available.
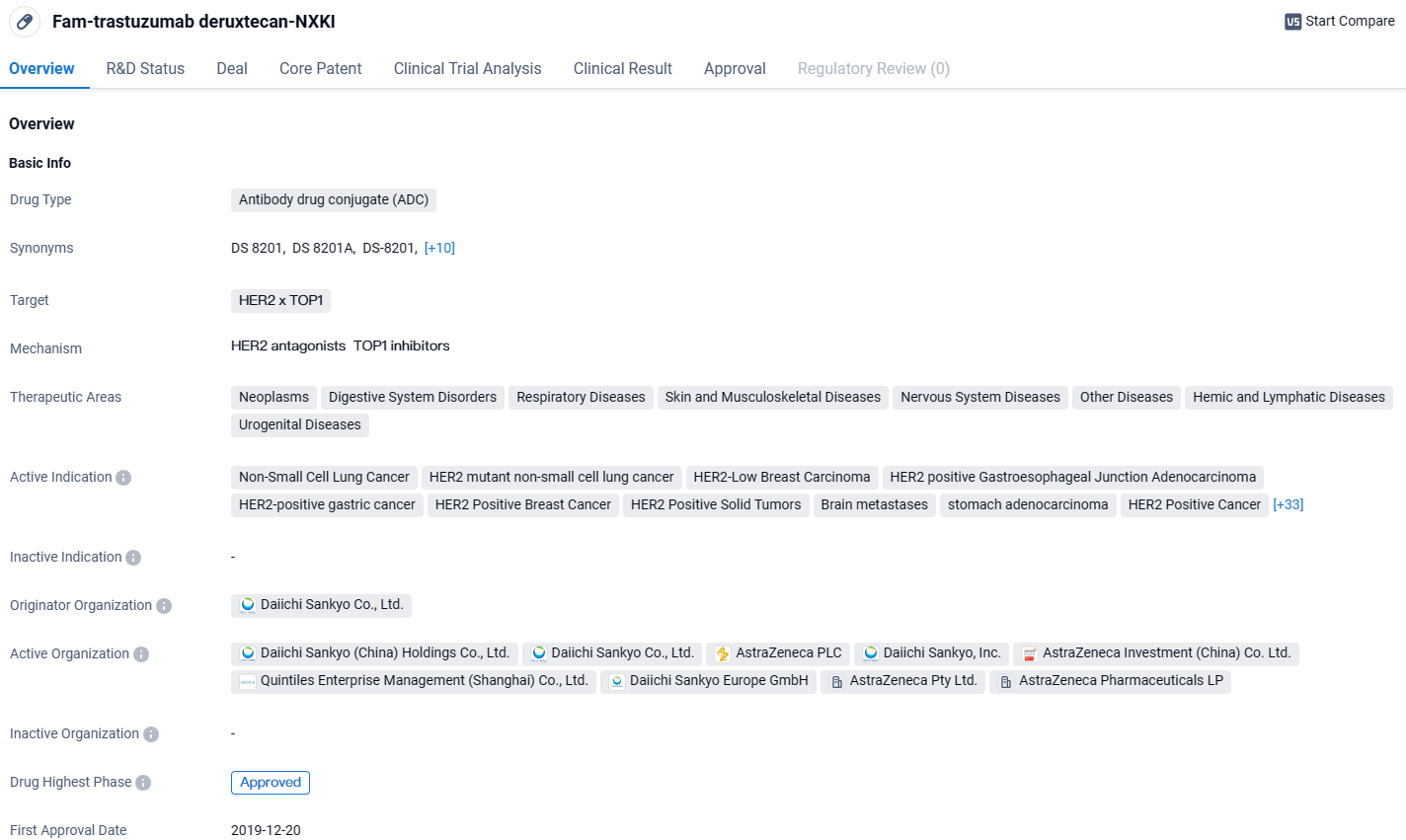
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The authorization for this specific use has been granted provisionally using an expedited process, anchored on measurable tumor shrinkage rates and the period those responses persist. The future status of this provisional approval could depend on the validation and detailed report of therapeutic advantages in a subsequent confirmatory research study.
The pioneer tissue-agnostic sanction for a directed HER2 therapy, which is also an ADC, derived from the analysis of effectiveness data gathered from a cohort of 192 adults with advanced, unmanageable, or metastatic solid tumors that exhibited positivity for HER2. These participants were incorporated into one of three separate multicenter phase 2 investigations, as part of the expansive DESTINY clinical research initiative, specifically DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02. The primary effectiveness metric utilized in each of the three analyses was the verified ORR while DOR served as an additional measure of effectiveness.
The authorization occurred subsequent to an assessment by the U.S. Food and Drug Administration utilising the Real-Time Oncology Review initiative, and in a context of both Expedited and Breakthrough Therapy Evaluations. The regulatory submission was concurrently appraised as a component of Project Orbis, establishing a collaborative international mechanism for the synchronized application and examination of cancer treatments among allied international regulatory authorities. Within the Project Orbis framework, regulatory bodies in Australia, Brazil, and Singapore are also examining ENHERTU for an identical medical indication.
Funda Meric-Bernstam, MD, the chairperson for Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center, highlighted, "Up until the sanctioning of trastuzumab deruxtecan, individuals suffering from metastatic solid tumors with a positive expression of HER2 were facing a scarcity of therapeutic alternatives." She added, "Observing the significant rates of clinical response across various trials, this approval of a tissue-agnostic therapy implies the availability of a HER2-focused therapeutic option for patients.”
Dave Fredrickson, Executive Vice President of the Oncology Business Division at AstraZeneca, remarked, "Being the initial ADC acknowledged for a tissue-agnostic use, ENHERTU is genuinely realizing its therapeutic promise for metastatic tumors susceptible to HER2." He also noted, "This decision emphasizes the significance of biomarker assessments, including HER2, across diverse tumor categories. This ensures that patients with advanced-stage cancers - with limited choices available- can determine the suitability of a directed therapy."
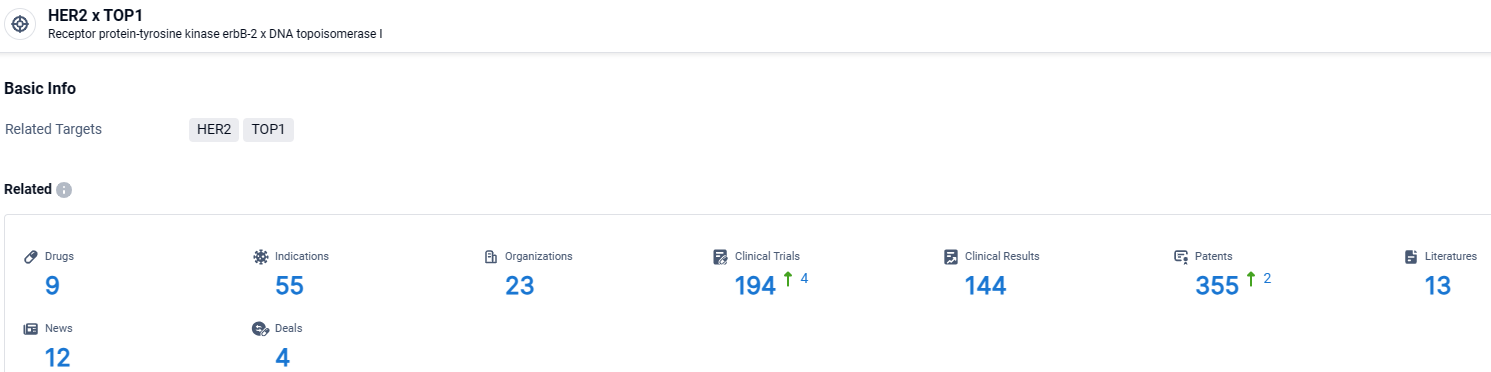
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 7, 2024, there are 9 investigational drugs for the HER2 and TOP1 target, including 55 indications, 23 R&D institutions involved, with related clinical trials reaching 194, and as many as 355 patents.
Fam-trastuzumab deruxtecan-NXKI is an ADC drug that targets HER2 and TOP1 and has been approved for various therapeutic areas and active indications. Its approval in the United States and China signifies its efficacy and safety in treating a wide range of diseases, particularly in the field of oncology.