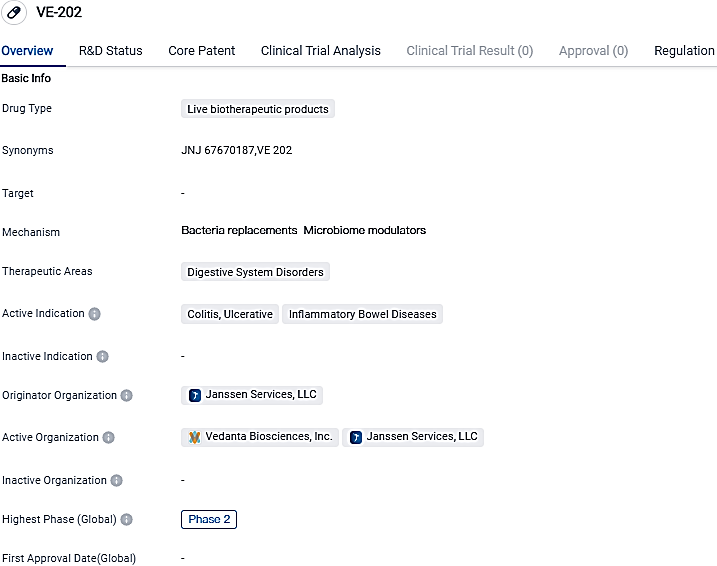
Vedanta Biosciences Reports Initiation of Phase 2 l Study for VE202 in Ulcerative Colitis
Vedanta Biosciences, a clinical-phase corporation specializing in the possible development of novel oral treatments grounded on defined bacterial consortia, recently reported the initiation of treatment for the initial participant in the COLLECTiVE202 Phase 2 investigation of VE202.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Vedanta notified that the U.S. FDA has bestowed Fast Track status to their bacterial consortium candidate, VE202, specified for ulcerative colitis treatment. Fas Track status aims to expedite the review and speed up the trials of drugs developed to address serious illnesses and satisfy an unmet medical demand.
"Despite the fact that nearly 50% of inflammatory bowel disease sufferers may be in remission at various times, chronic monitoring indicates the majority could relapse eventually. New ulcerative colitis treatments may be effective for many, but often come with safety concerns, such as the risk of infection", stated by Jeffrey Silber, M.D., Vedanta Biosciences' Chief Medical Officer.
Jeffrey Silber continued, "We are pleased that the FDA's Fast Track status for VE202. We speculate this candidate could present ulcerative colitis patients with a different treatment option, proposing a promising safety outlook. We eagerly anticipate the progression of this program as we endeavor to meet a significant unmet medical demand."
Following vancomycin pre-treatment, VE202 strains populated significantly and persistently in a Phase 1 trial with healthy volunteers, following both dosage- and time-dependent manner. Alongside this, VE202 boosted the alteration of primary bile acids into secondary bile acids that immunomodulate and counter intestine inflammation compared to a placebo. VE202 was also well-received, with no serious adverse effects related to treatment.
The COLLECTiVE202 trial is a randomized, double-blind, placebo-controlled study being conducted in the U.S. and Europe. It intends to enroll 100 ulcerative colitis patients with mild to moderate status, aged between 18 and 75. Either a placebo or VE202 is added to the existing treatment and Vedanta will review two varying methods, giving all participants the chance to receive VE202. Safety and endoscopic responses are the primary goals; secondary goals encapsulate clinical response and remission, alongside other endoscopic, histologic, colonization, inflammatory, immune biomarkers, and quality-of-life measures.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
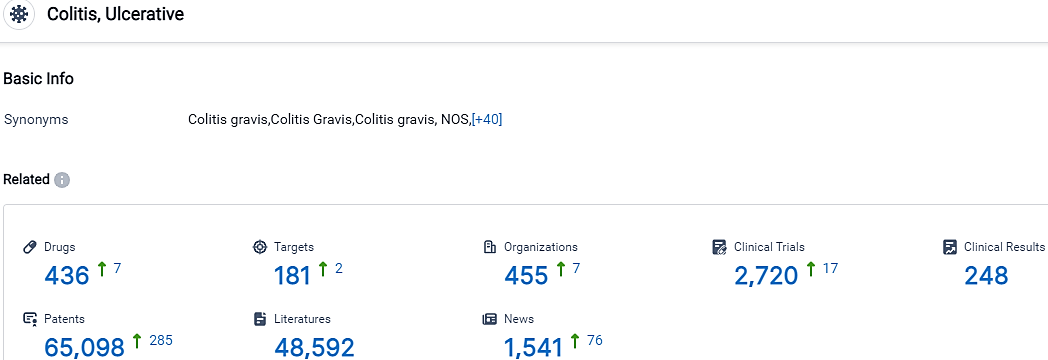
According to the data provided by the Synapse Database, As of October 14, 2023, there are 436 investigational drugs for the Ulcerative Colitis, including 181 targets, 455 R&D institutions involved, with related clinical trials reaching 2720,and as many as 65098 patents.
VE202 is a first-in-class, orally administered, investigational live biotherapeutic product consortium consisting of 16 strains of bacteria, which were rationally selected to induce immune tolerance in the gut, reverse the gut microbiota abnormalities that are common in patients with inflammatory bowel disease, and strengthen the epithelial barrier. VE202 was granted Fast Track designation in 2023 by the U.S. FDA for the treatment of UC.