Vicebio Advances RSV/hMPV Vaccine Trials and Strengthens Board with Industry Experts
Vicebio Ltd (“Vicebio”), a biopharmaceutical firm at the forefront of developing next-generation vaccines for severe respiratory viruses, has disclosed updates regarding the ongoing Phase 1 (P1) study of its VXB-241 bivalent RSV-hMPV. Additionally, the company has appointed Moncef Slaoui, PhD, and Khurem Farooq to its Board of Directors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
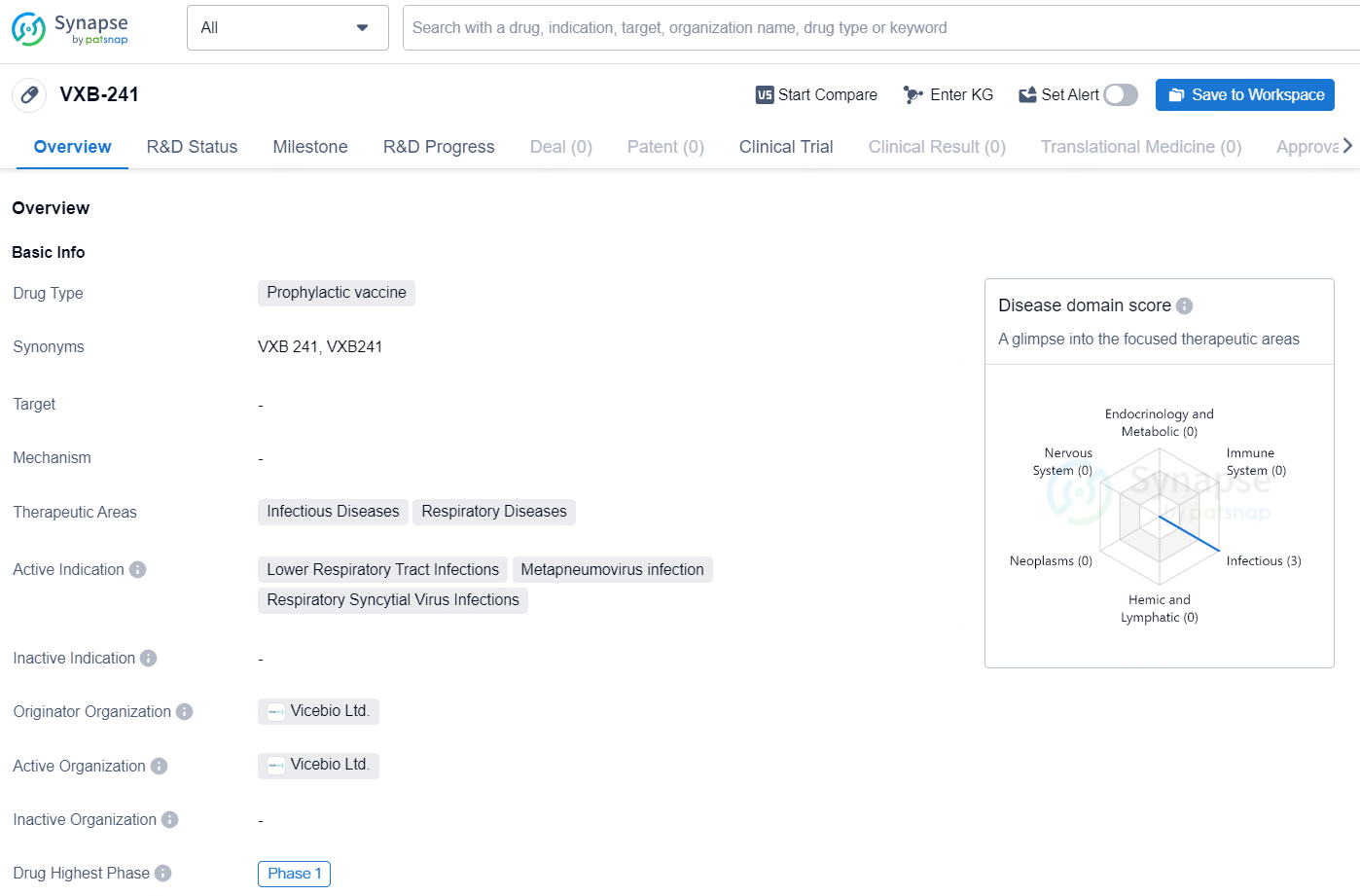
Vicebio is entering the second phase of its clinical trial for VXB-241 and is currently recruiting participants aged 60 and older to assess the safety of the candidate vaccine in this high-risk group. Initial results from the trial are expected by mid-2025, which Vicebio believes will be a key achievement in validating its clinical platform. Additional details regarding the Phase 1 study can be accessed on clinicaltrials.gov under the identifier NCT06556147.
Moncef Slaoui, PhD, and Khurem Farooq have joined Vicebio's Board of Directors, effective immediately, bringing extensive expertise in vaccine development and leadership within the biopharmaceutical industry.
Emmanuel Hanon, the CEO of Vicebio, remarked: “As part of our significant Series B funding, we are enhancing our top-tier leadership team with Moncef and Khurem's additions to the Board. Their knowledge will not only bolster our innovative efforts but is also vital as we continue to pursue the company's goal of delivering next-generation respiratory viral vaccines.”
Central to Vicebio's innovations is its exclusive Molecular Clamp technology, which aims to stabilize viral antigens while improving immune protection against various respiratory pathogens. This groundbreaking technology facilitates the creation of ready-to-use, cost-efficient multivalent respiratory viral vaccines that address urgent needs in global respiratory health.
Moncef Slaoui, PhD, the newly appointed Independent Board Member at Vicebio, expressed: “The Molecular Clamp technology from Vicebio represents a significant leap forward in vaccine development. It is thrilling to join the Board of a firm where this innovative technology has the potential to accelerate the availability of highly effective and stable vaccines in prefilled syringes.”
Khurem Farooq, also a newly appointed Independent Board Member at Vicebio, stated: “Vicebio is experiencing an exciting growth phase as it aims to advance its pipeline of multivalent vaccines capable of preventing a broad spectrum of diseases with a single vaccine."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
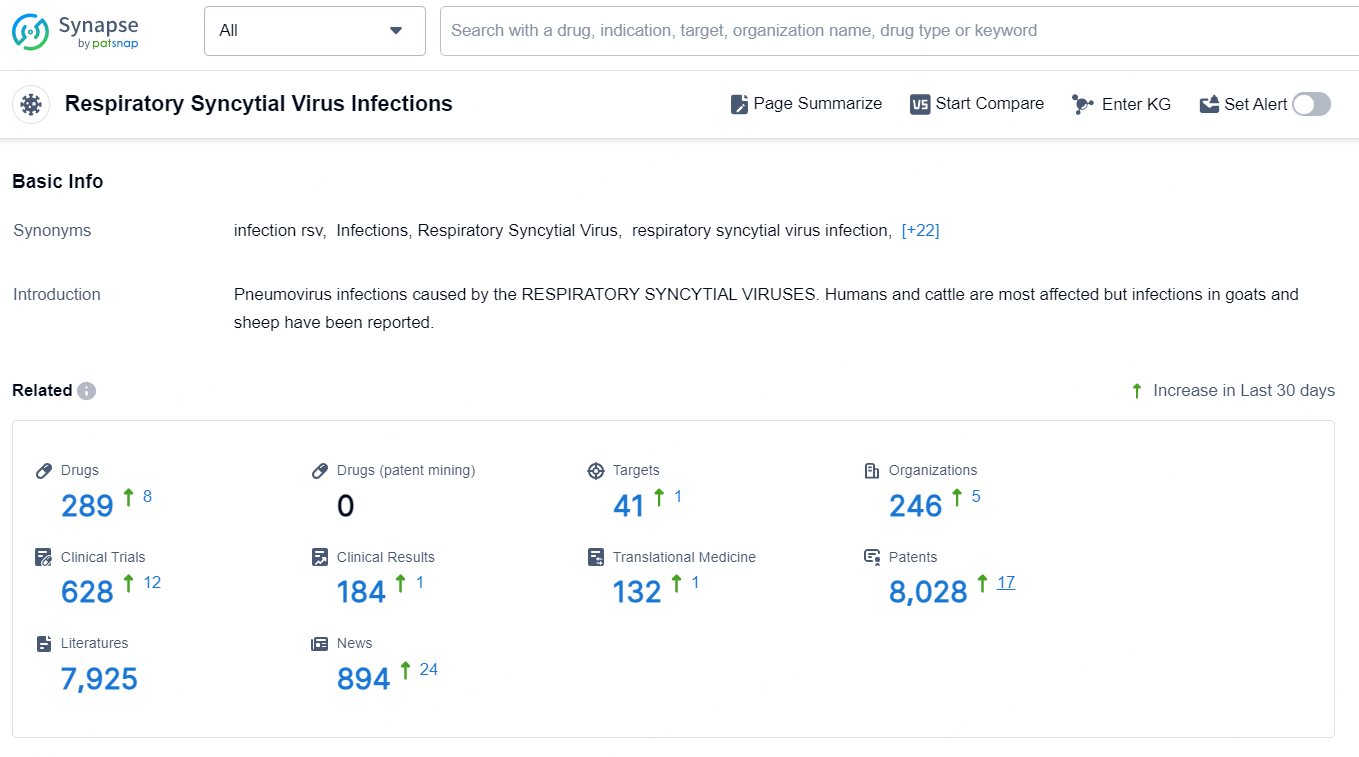
According to the data provided by the Synapse Database, As of November 27, 2024, there are 289 investigational drugs for the Respiratory Syncytial Virus Infections, including 41 targets, 246 R&D institutions involved, with related clinical trials reaching 628, and as many as 8028 patents.
VXB-241 is a prophylactic vaccine currently in development by Vicebio Ltd. The drug is being studied for its potential use in the treatment of lower respiratory tract infections, metapneumovirus infection, and respiratory syncytial virus infections. These conditions fall within the therapeutic areas of infectious diseases and respiratory diseases, highlighting the potential impact of VXB-241 on addressing these significant health issues.