Vivet Therapeutics Begins Phase 1/2 Trial Dosage for Second Group in Wilson Disease Study
Vivet Therapeutics, an innovative biotechnological enterprise engaged in the advancement of groundbreaking and persistent gene treatments for uncommon genetic metabolic diseases of the liver, has disclosed that it has administered the initial patient with its primary candidate VTX-801 as part of Cohort 2 in its active GATEWAY Clinical study aimed at combating Wilson Disease. The commencement of Cohort 2 comes on the heels of the satisfactory conclusion of Cohort 1 and the subsequent endorsement by a standalone Data Supervision Panel to advance.
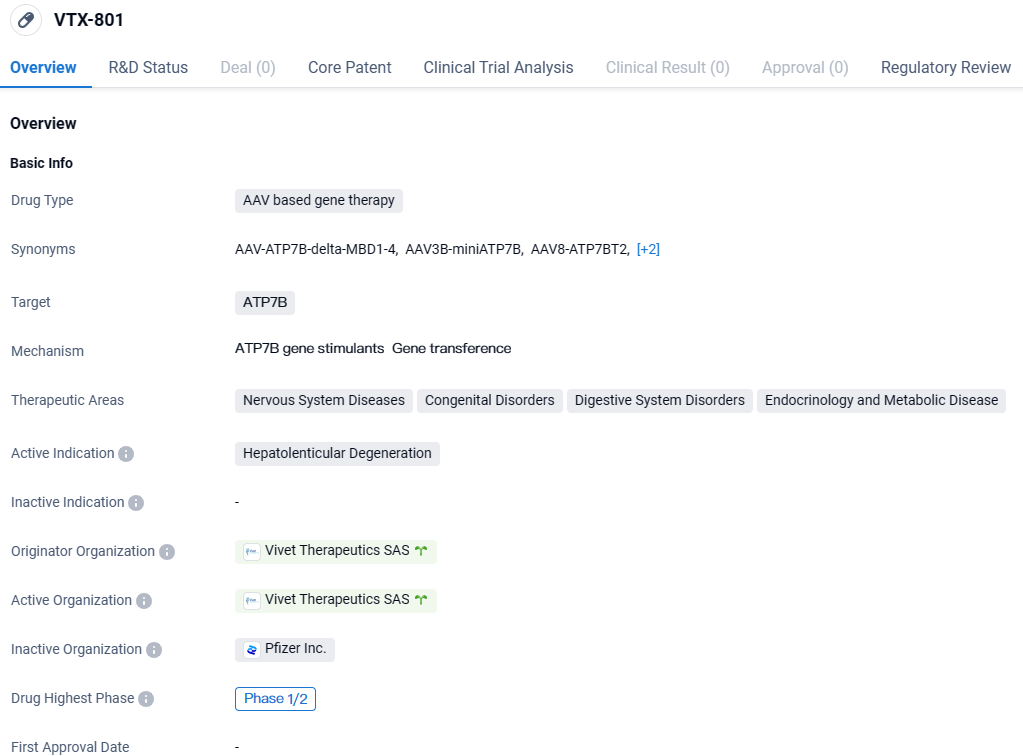
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Wilson's Disease (WD) is a pathological condition that results in the excessive accumulation of copper within the liver and brain, leading to significant neurologic and liver-related health issues. Current research suggests that the incidence of premature mortality in WD patients is significantly increased—by a factor of 2 to 4 times—compared to the average population, in spite of available treatments that meet the standard of care. Globally, WD impacts around one in every 30,000 individuals, with a prevalence of about 12,000 to 15,000 cases in the United States and nearly 17,000 cases in Europe.
The GATEWAY clinical trial remains in progress, as it continually welcomes new participants. This non-randomized, open-label, Phase 1/2 study is conducted in various clinical institutions in the United States, the United Kingdom, Germany, and Denmark. The study's main objective is to determine the safety profile, acceptable dosage limits, and pharmacological efficacy of an isolated intravenous dosage of VTX-801 in escalating doses for adults affected by Wilson's Disease.
Jean-Philippe Combal, the CEO of Vivet Therapeutics, commented: "The endorsement from the Data Monitoring Committee (DMC) to proceed with a greater dosage is a promising development and propels our efforts to refine the clinical progression of VTX-801. The initial data regarding safety, histological impact, and pharmacodynamics behavior shown by VTX-801 is promising. We are gratified to report that we’ve successfully administered the product to the initial participant in the second cohort of the GATEWAY trial."
After the green light from the DMC, Vivet has escalated the dosage for the initial patient in the second cohort. This decision was informed by the promising data regarding patient safety and drug tolerance, as well as the preliminarily observed pharmacodynamic effects in the first two participants from Cohort 1. There were no surprising adverse events linked to the treatment at the beginning of Cohort 1's dosage level.
Continual observation of patients in Cohort 1 has revealed a steady rise in ceruloplasmin enzyme activity and the presence of ATP7B minigene mRNA in the liver, with one biopsy at the one-year mark indicating histological improvements in the liver. Nonetheless, due to certain findings from a specialized copper biomarker test, the two patients have been categorized as having an inadequate response, and therefore, their standard-of-care treatments remain ongoing. Additional findings from the GATEWAY trial are scheduled for presentation at various scientific meetings in the year 2024.
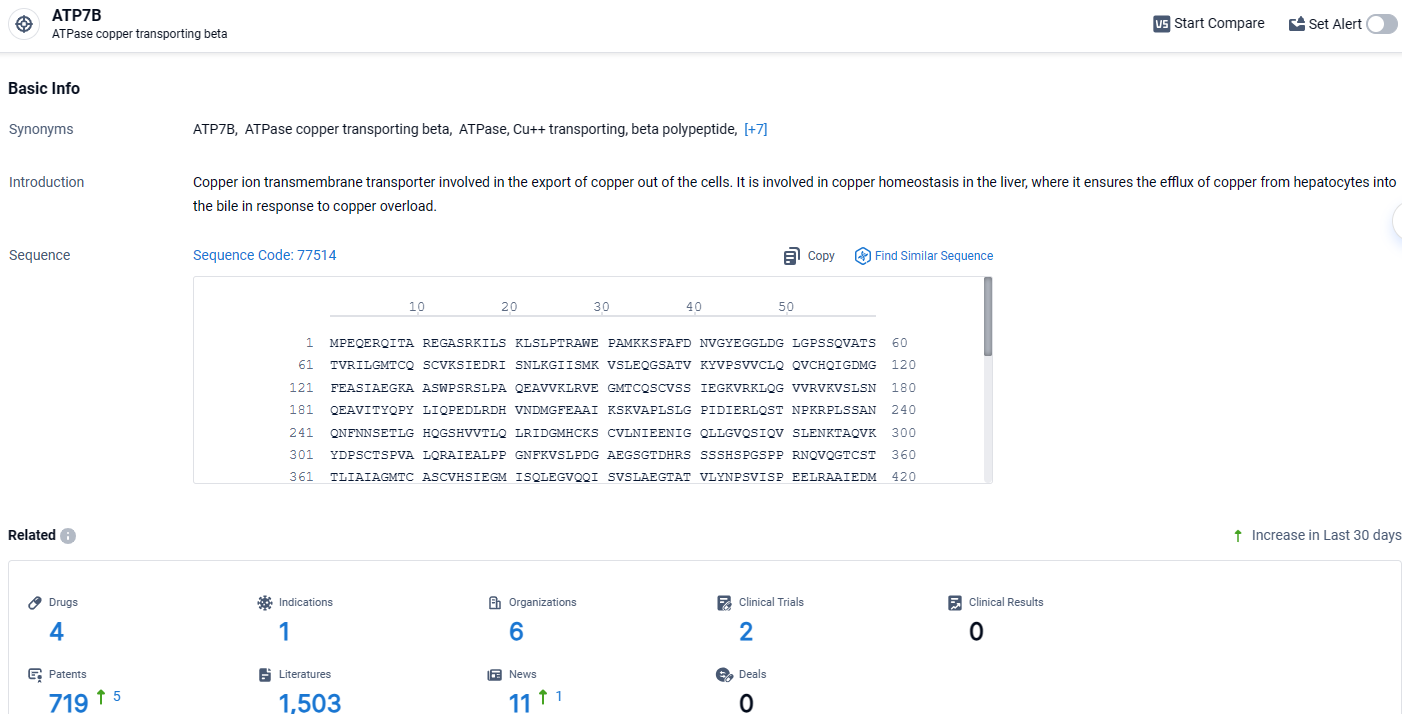
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 10, 2024, there are 4 investigational drugs for the ATP7B target, including 1 indications, 6 R&D institutions involved, with related clinical trials reaching 2, and as many as 719 patents.
VTX-801 is an AAV-based gene therapy drug developed by Vivet Therapeutics SAS. It targets ATP7B and is being developed for the treatment of hepatolenticular degeneration. The drug has shown promising results in early studies and is currently in Phase 1/2 of development. It has been granted Fast Track and Orphan Drug designations, indicating its potential to address unmet medical needs.