What ADC-related progress will Lepu Biopharma reveal at the ASCO conference?
Lepu Biopharma is an innovative biopharmaceutical company focused on the field of oncology treatment, particularly in targeted therapy and immunotherapy. It is dedicated to developing innovative ADCs through an advanced ADC technology development platform, creating optimized and novel medications to better meet the unmet clinical needs of cancer patients. Moreover, the company is developing combination therapies of IO+ADC utilizing its marketed PD-1 monoclonal antibody, Pucotenlimab, taking full advantage of pipeline synergy. Additionally, Lepu Biopharma has expanded and diversified its pipeline by introducing oncolytic virus products through partnerships.
At this year's ASCO, Lepu Biopharma presented three studies, with two clinical research findings delivered orally; these included the ADC drugs MRG003, MRG004A, and the PD-1 antibody Pucotenlimab (HX008).
1.MRG003: MRG003 is an ADC composed of an EGFR-targeting monoclonal antibody linked to the potent microtubule inhibitor payload MMAE via a vc linker. It specifically binds to EGFR on the surface of tumor cells with high affinity and, upon internalization into the tumor cell, it releases its potent payload, leading to tumor cell death. Previously, MRG003 was granted Fast Track Designation (FTD) by the U.S. Food and Drug Administration (FDA) for the treatment of advanced nasopharyngeal carcinoma (NPC) and was recognized by the CDE as a breakthrough therapy drug (BTD). Currently, a registrational Phase IIb trial for MRG003 targeting NPC has completed enrollment and is expected to apply for marketing approval in 2024; a Phase III trial for head and neck squamous cell carcinoma (HNSCC) is ongoing; indications for NPC and HNSCC have been granted IND approval in the USA.
In a Phase IIa trial targeting NPC, patients who had failed or were intolerant to at least one platinum-based regimen and PD-1/L1 inhibitor treatment received MRG003 at doses of 2.0 mg/kg (n=30) or 2.3 mg/kg (n=31). The primary endpoint was the Overall Response Rate (ORR) assessed by RECIST 1.1 criteria, while secondary endpoints included Disease Control Rate (DCR), Duration of Response (DoR), Progression-Free Survival (PFS), and safety. As of March 15, 2023, the 2.0 mg/kg dose group had 28 assessable patients, showing an ORR of 39.3%, a DCR of 71.4%, a median DoR of 6.8 months, and a median PFS of 7.3 months. In the 2.3 mg/kg dose group, 29 patients were assessable, with an ORR of 55.2%, DCR of 86.2%, median DoR of 6.8 months, and immature median PFS. Regarding safety, the most common treatment-related adverse events (TRAEs) included rash (49.2%), itching (41.0%), anemia (34.4%), and hair loss (31.1%), with most TRAEs being Grade 1 or 2 according to the CTCAE 5.0 standards. The rate of treatment-related severe adverse events (SAE) was 11.5% (7/61). Dose reductions due to TRAEs occurred in 13.1% (8/61) of patients, and treatment was discontinued in 3 patients (4.9%). No treatment-related deaths were observed.
2. MRG004A: MRG004A is an innovative ADC targeting tissue factor (TF) and is obtained by site-specific conjugation. It employs a differentiated design, selecting a humanized anti-TF monoclonal antibody with high TF affinity for rapid internalization, which is observable within one hour and minimizes the impact on coagulation. This drug has been granted Orphan Drug Designation and Fast Track Designation by the FDA. Preclinical studies in multiple TNBC, ovarian cancer, and PDAC CDX and PDX models have demonstrated the in vivo anti-tumor activity of MRG004A. Currently, MRG004A is undergoing Phase I/II clinical trials in the United States and China, and anti-tumor activity signals have been observed in indications such as pancreatic cancer, triple-negative breast cancer, and cervical cancer.
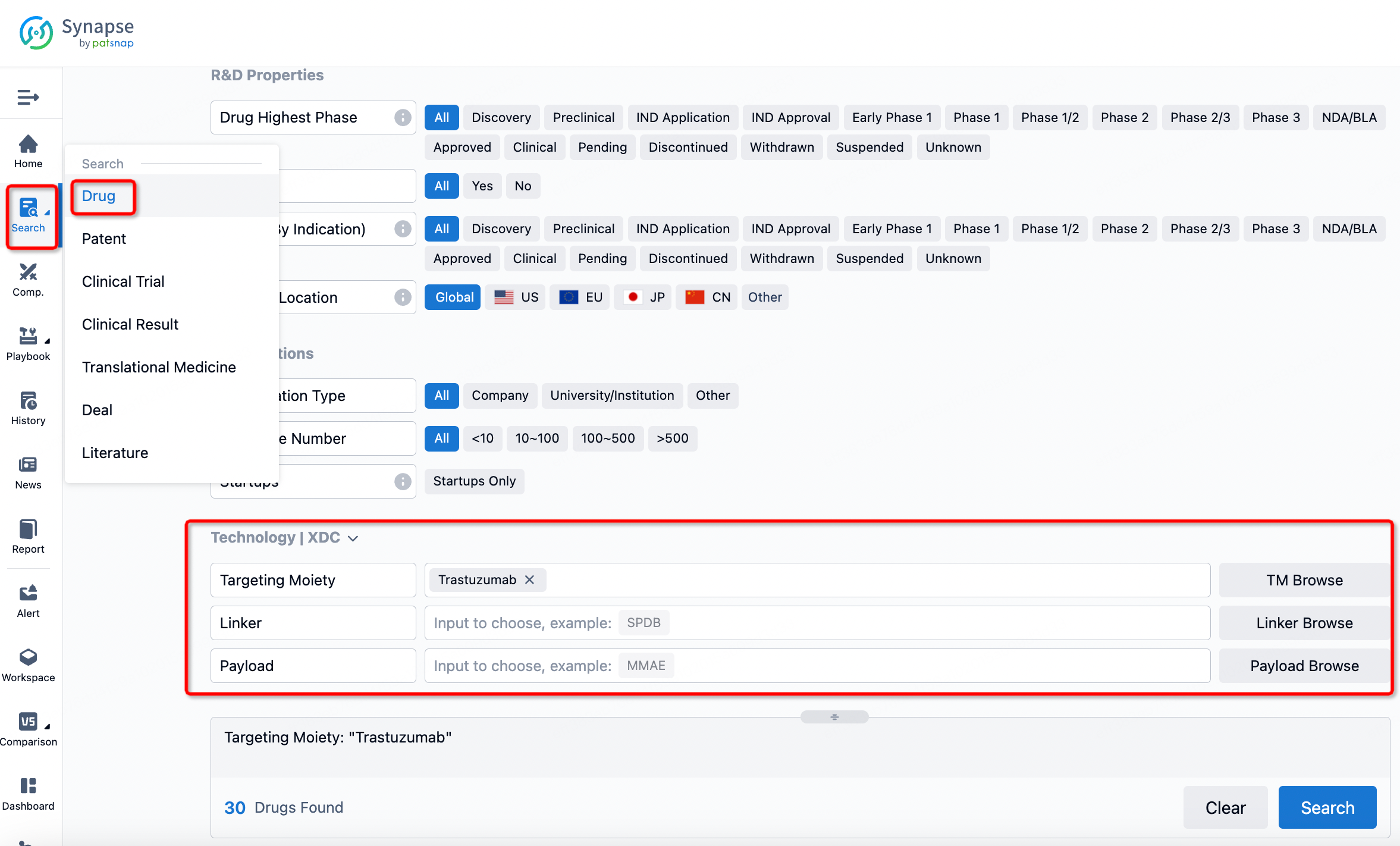
How to search for and analyze the development progress of ADC pharmaceuticals?
If you want to learn about the latest developments in ADC drugs, you can use the drug search module of the Synapse database. This module supports searching for ADC drugs by classification through Targeting Moiety, Linker, and Payload.
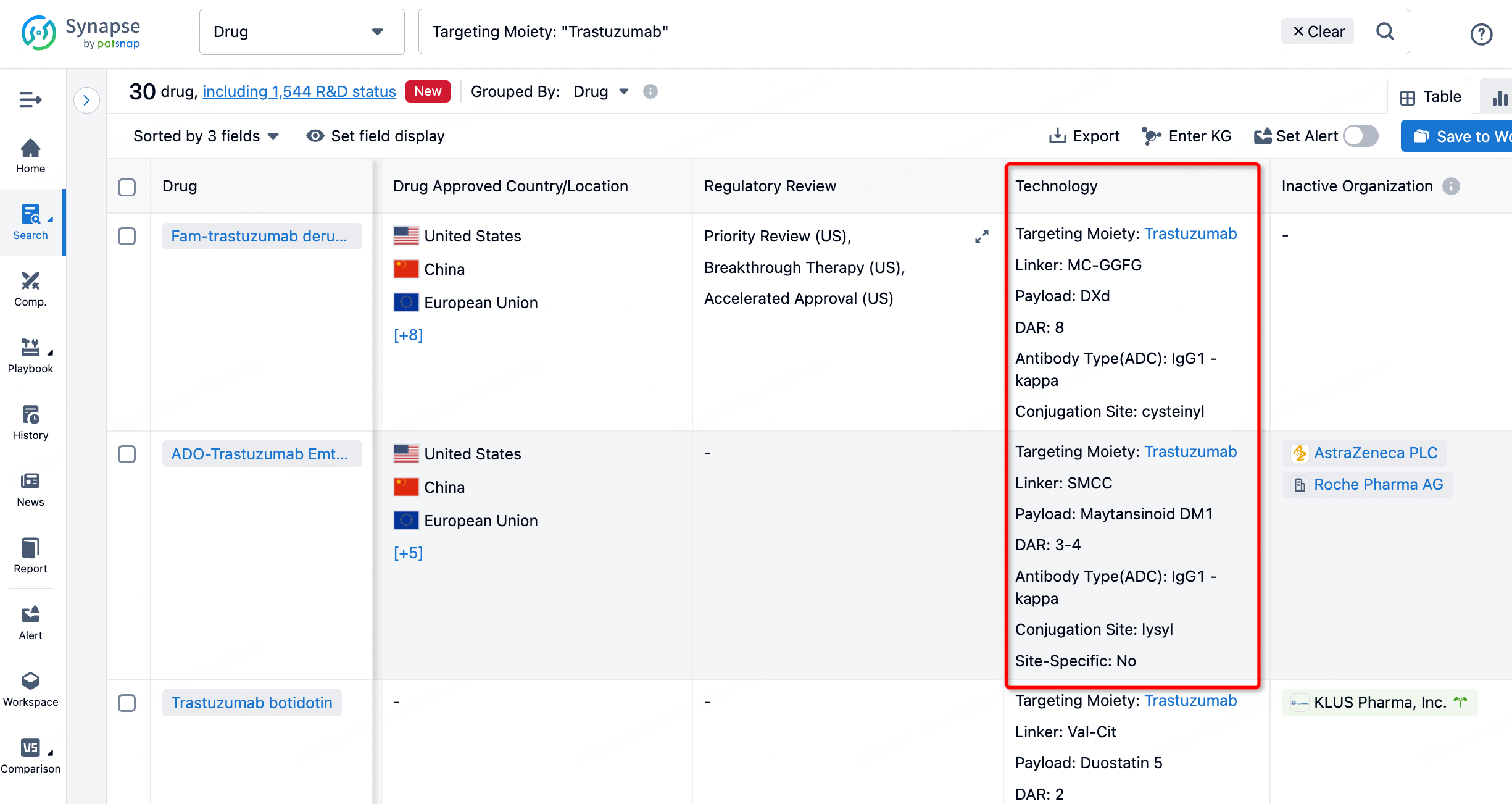
On the search results page, you can easily review information related to the ADC's technical category of the drugs.
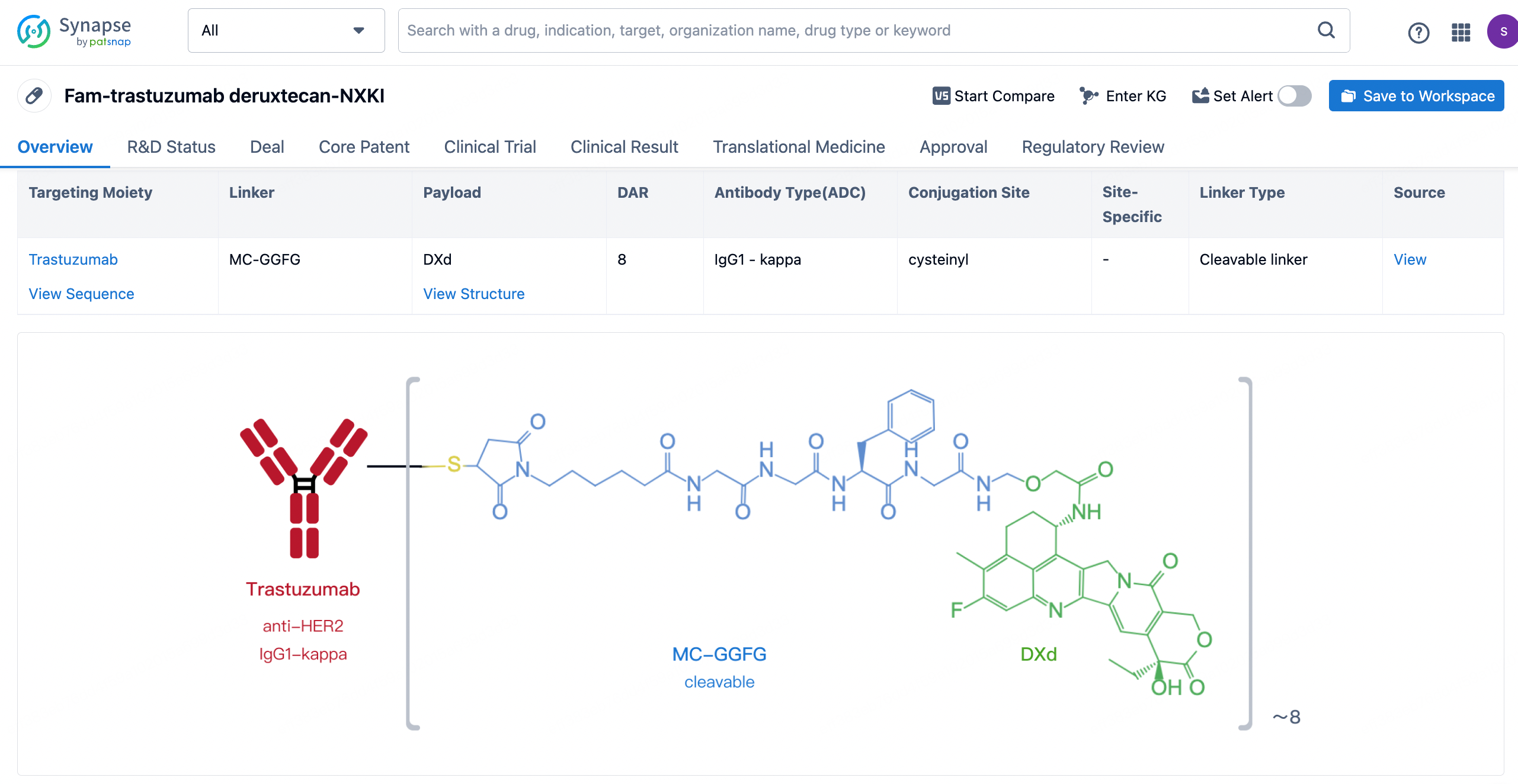
After clicking to enter the drug details page, you can also effortlessly obtain structural information about the ADC drug.
Click on the image below to embark on a brand new journey of drug discovery!