What Retrieval Methods Can be Used in the CAR-T Field to Find More Comprehensive Technical Materials and Patent Information? (Part I)
In the CAR-T field, we can perform comprehensive data retrieval of CAR-T in two major parts: antibody search and CAR expression cassette design.
Antibody Search: To enable CAR-T to reach its target successfully, it is essential to choose the appropriate antigen recognition domain.
CAR Expression Cassette: The design of the CAR expression cassette determines whether a T cell can become CAR-T, and the effect it produces.
In this article, we will focus on the antibody search part. Here are several ways to perform a data search:
1.Input the heavy and light chain sequences and their CDRs on the antibody search interface, and generate an antibody search report with one click;
2.Use Motif search to search for scFv sequences based on CDRs; if you need to define the variation of CDRs, customize CDRs according to wildcards and search for related sequences in the entire sequence library.
3.Drug/gene index search for antibody heavy/light chain sequences, quickly query their patents and publicly available literature.
Whether we need to protect the antibodies we design or look at some technical documents to design antibodies, the first step is to understand the way antibodies are represented in claims, including antibody CDRs; heavy (VH) and light (VL) variable regions of antibodies; antibody heavy chains (HC) and light chains (LC); the nucleic acid sequence encoding the antibody.
Next, let's look at the specific search methods:
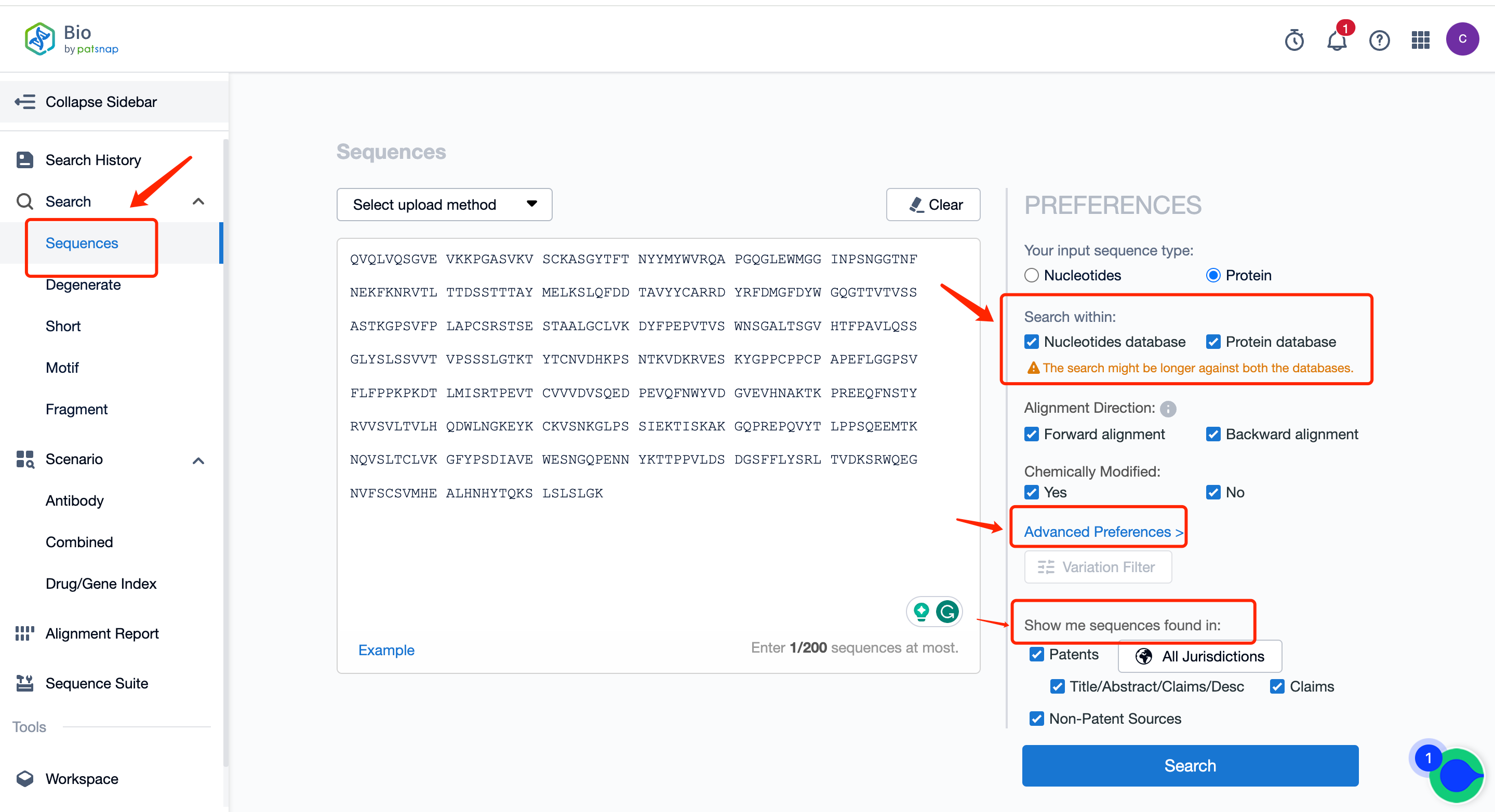
I. Conventional search can be conducted in the Bio database.
In a conventional search, both the protein and nucleotide databases are selected for searching, retrieving antibody sequences represented by protein sequence or nucleotide sequences encoding antibodies, and viewing their literature, patents, and other publicly disclosed information. The specific operation steps are as follows:
First of all, register for a free Patsnap Bio Sequence Database account. On the database's homepage, directly input the Keytruda heavy chain sequence in the conventional search and select both the nucleic acid sequence library (to search the nucleic acid sequence encoding the antibody sequence) and the protein sequence library. Simultaneously, in the advanced settings on the right, you can customize sequence similarity and target similarity based on patents. You can also customize the sequence position in the patents.
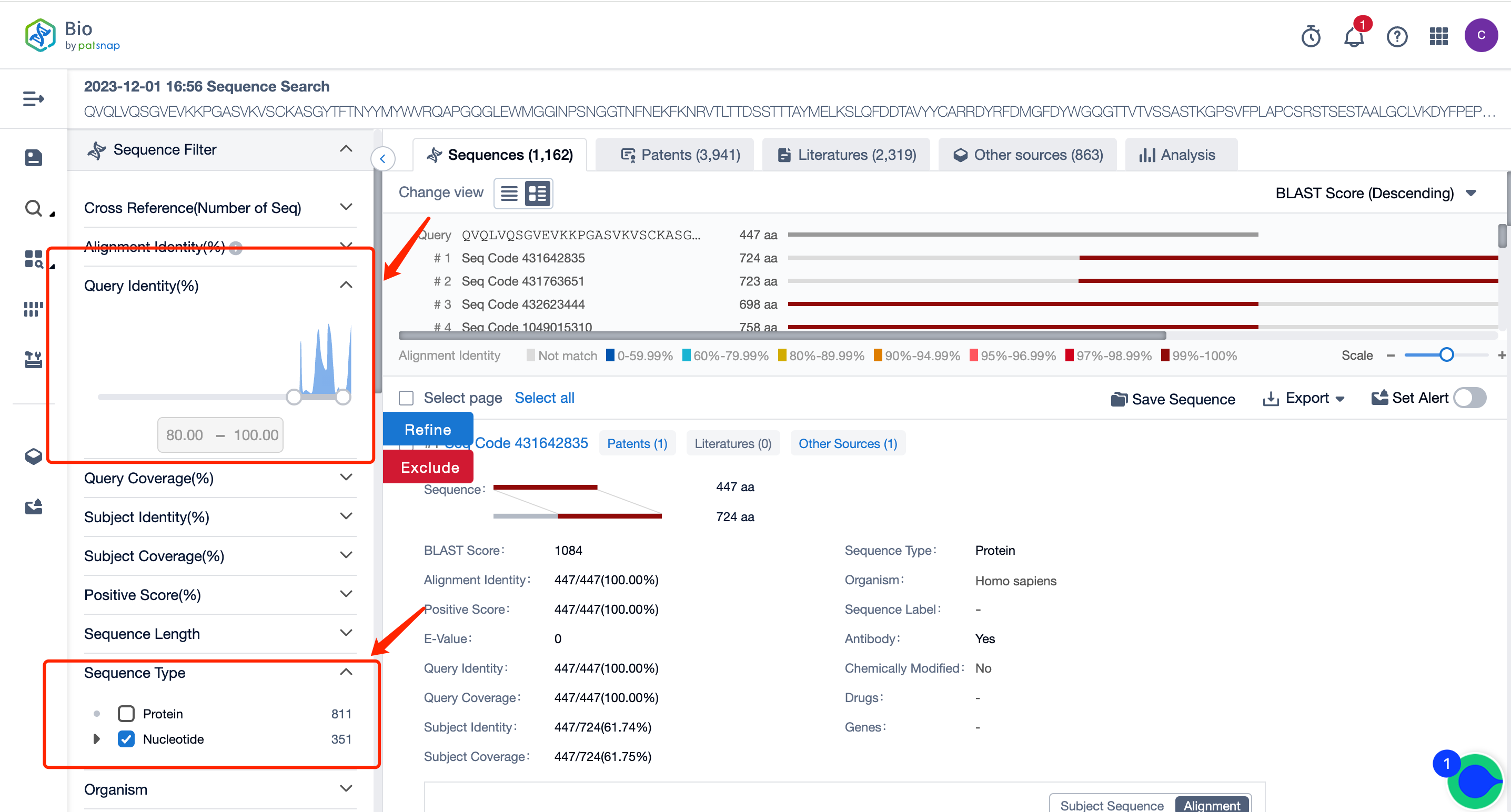
Through the search, we retrieved 1628 sequences. If the amount of data is excessive, you can use the filter items on the left to set conditions. For instance, we are setting the query similarity to above 80% and screening the nucleotide sequence type for viewing first.
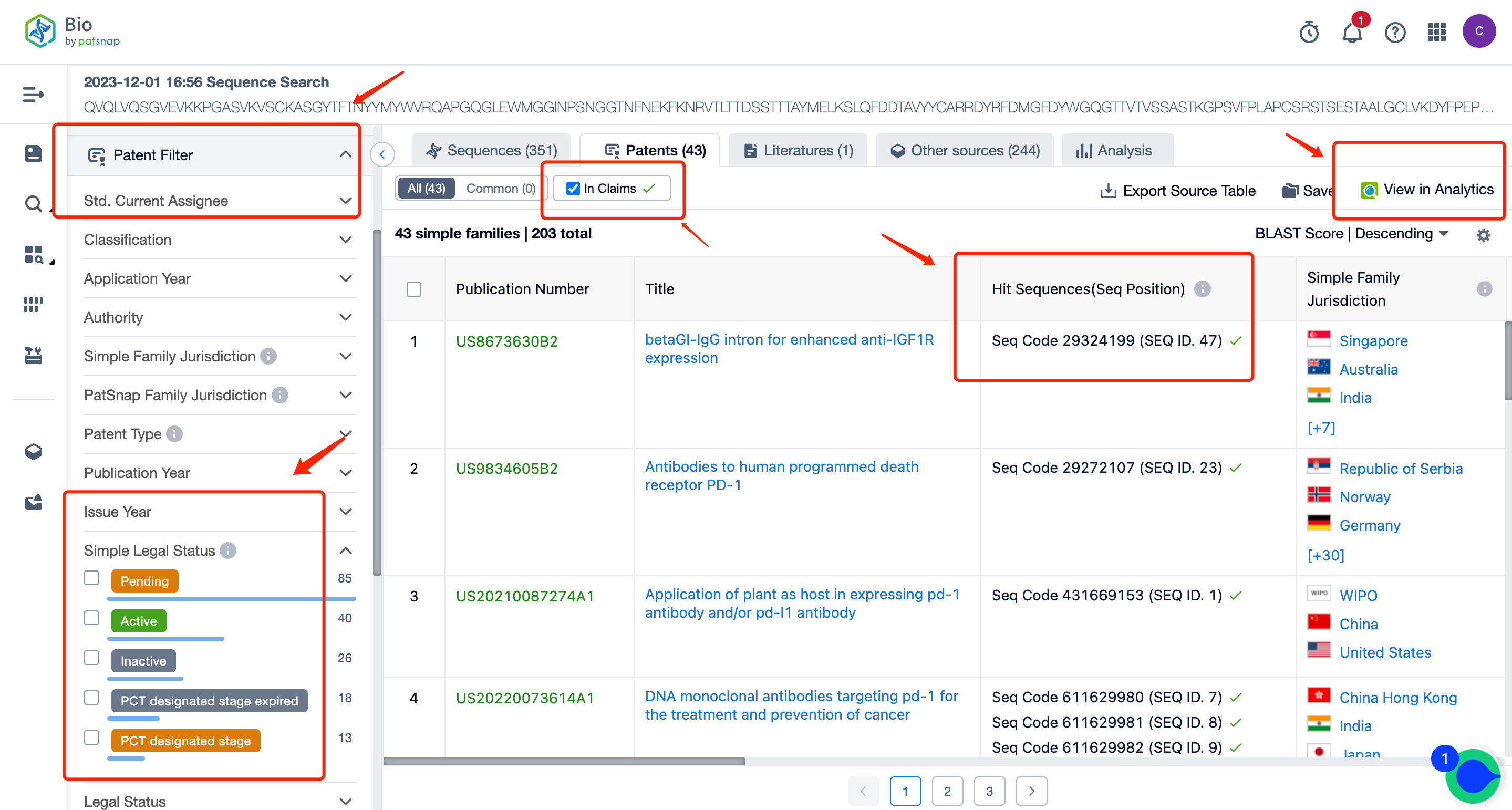
After filtering, the number of sequences is reduced to 351. On this result page, you can directly view all the patent data. You can also select to only view patents with sequences disclosed in the claims, and in the patent filter items on the left, you can filter and select patents according to the applicant, legal status, etc. You can directly click on the patent to go to the patent database for analysis.
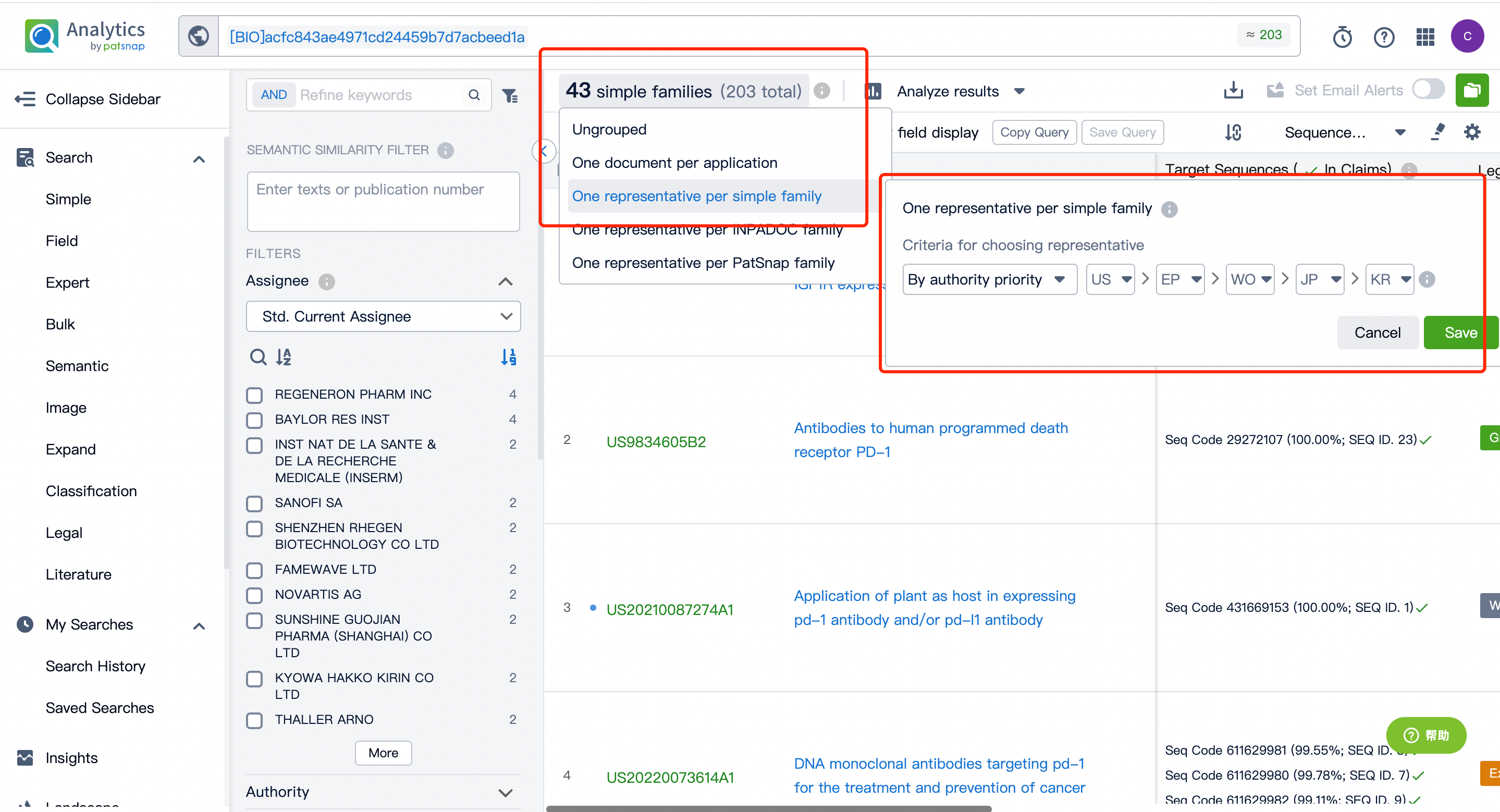
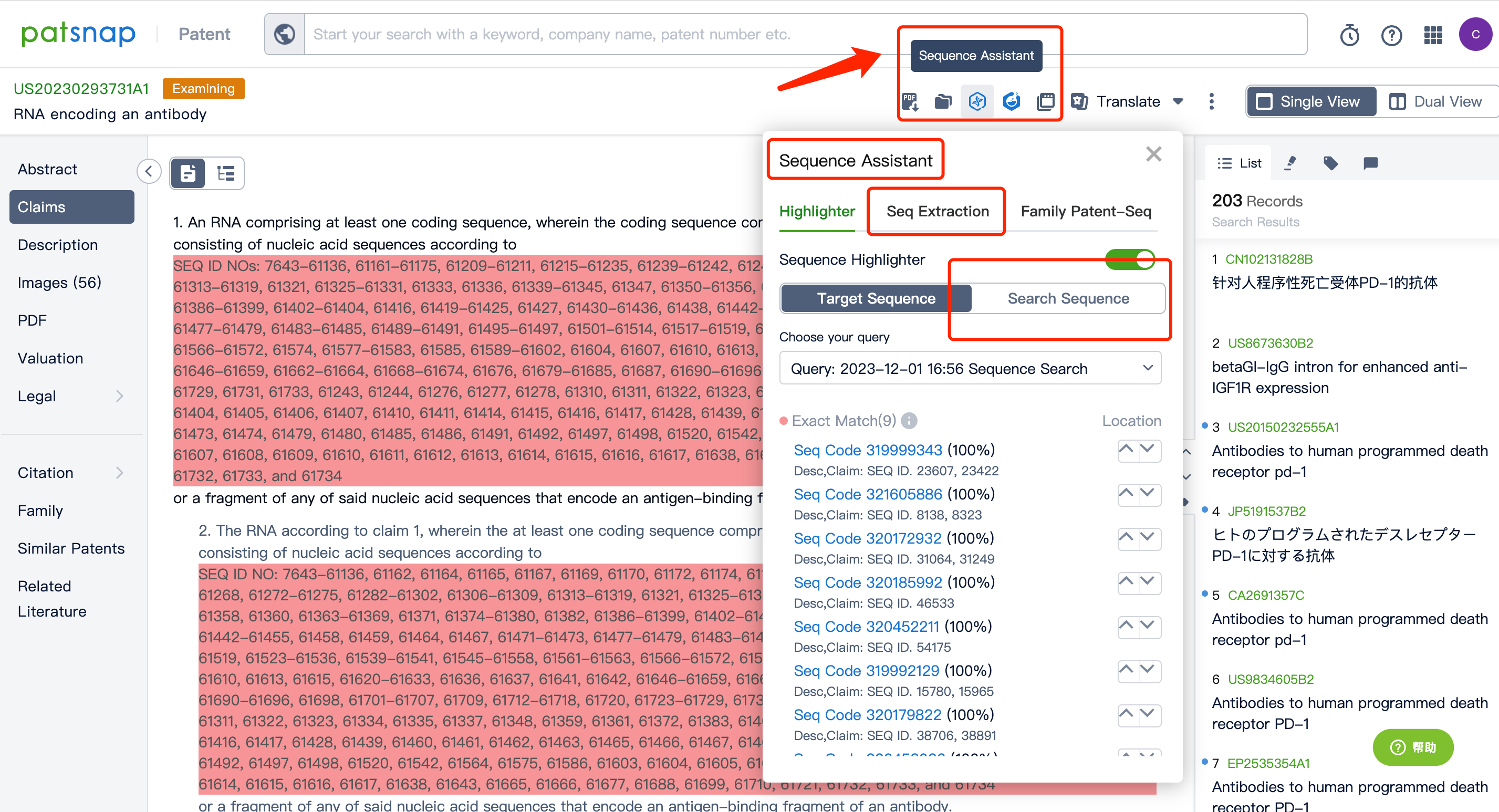
After entering the patent database, we can do a simple consolidation of the same family patents according to the publication date or acceptance bureau. After that, by clicking to enter the patent details, we can use the sequence assistant to locate the precise match or similar match of the Query sequence in the patent. You can also customize the search sequence in the current patent and can further extract the sequence FASTA to the local or extract it to the Bio database.
II. In the Bio database's antibody search, you can generate an antibody search report with one click.
Antibody Search: Input antibody light and heavy chain sequences and CDRs to generate an antibody search report which allows the inspection of the following: similar sequences of antibody light and heavy chains, antibody sequences containing said CDRs, patents, literature related to antibodies characterized by CDRs, CDR mutations, etc. The specific operation steps are as follows:
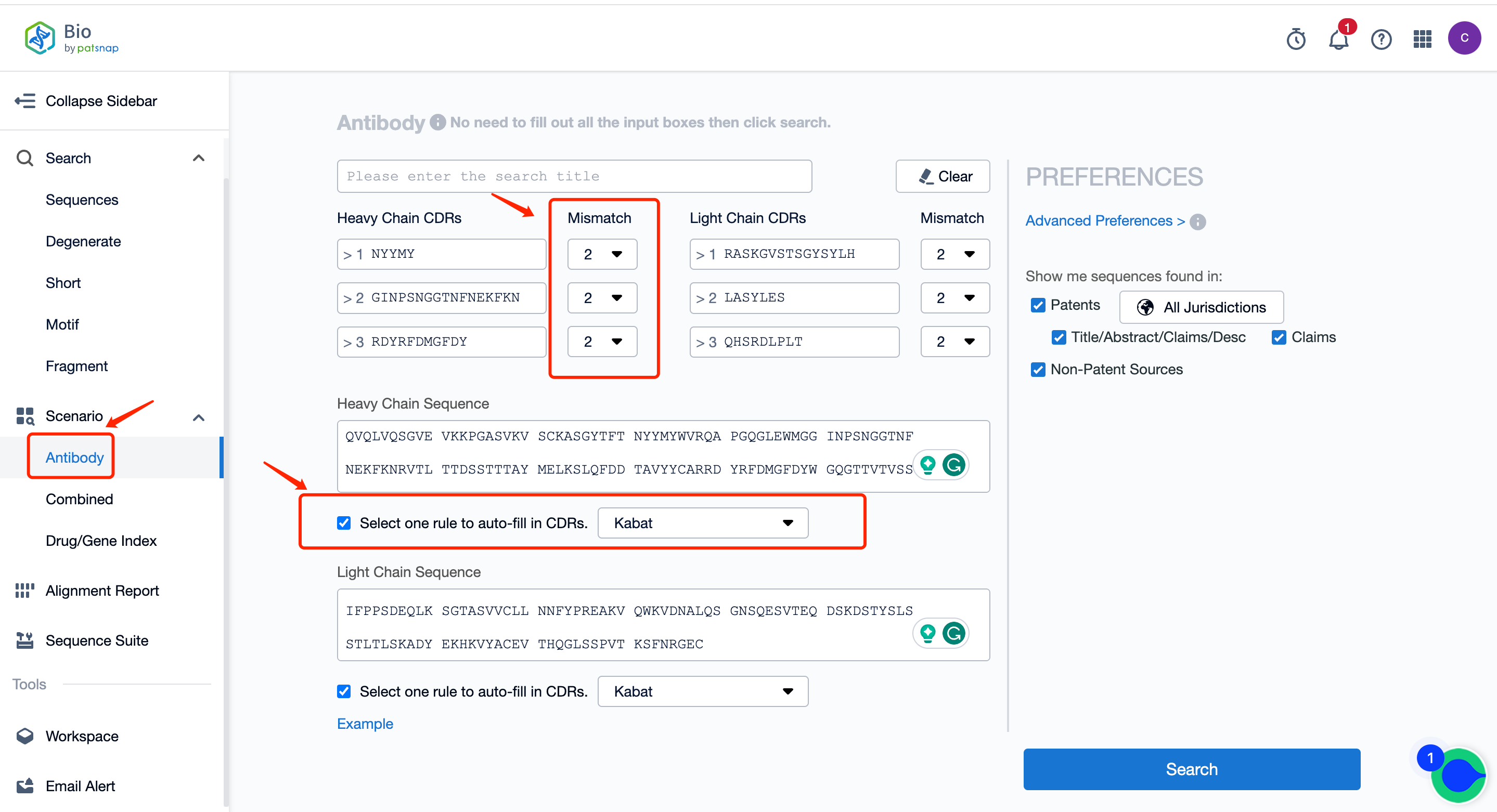
First of all, register a Patsnap Bio sequence database account, go to the antibody section on the homepage scene, input the heavy and light chains of the antibody to be searched, select one rule (IMGT, Kabat, AbM, Chothia, Contact) to automatically fill in the CDRs and fill in the mismatch number. After setting, search to generate an antibody search report.
In the report, one can initially observe the status of the number of heavy-light chain and CDRs hit sequences as well as their public source quantity. Following this, the matching conditions of the heavy chain CDRs, light chain CDRs, and the detection of CDR variations can also be observed.
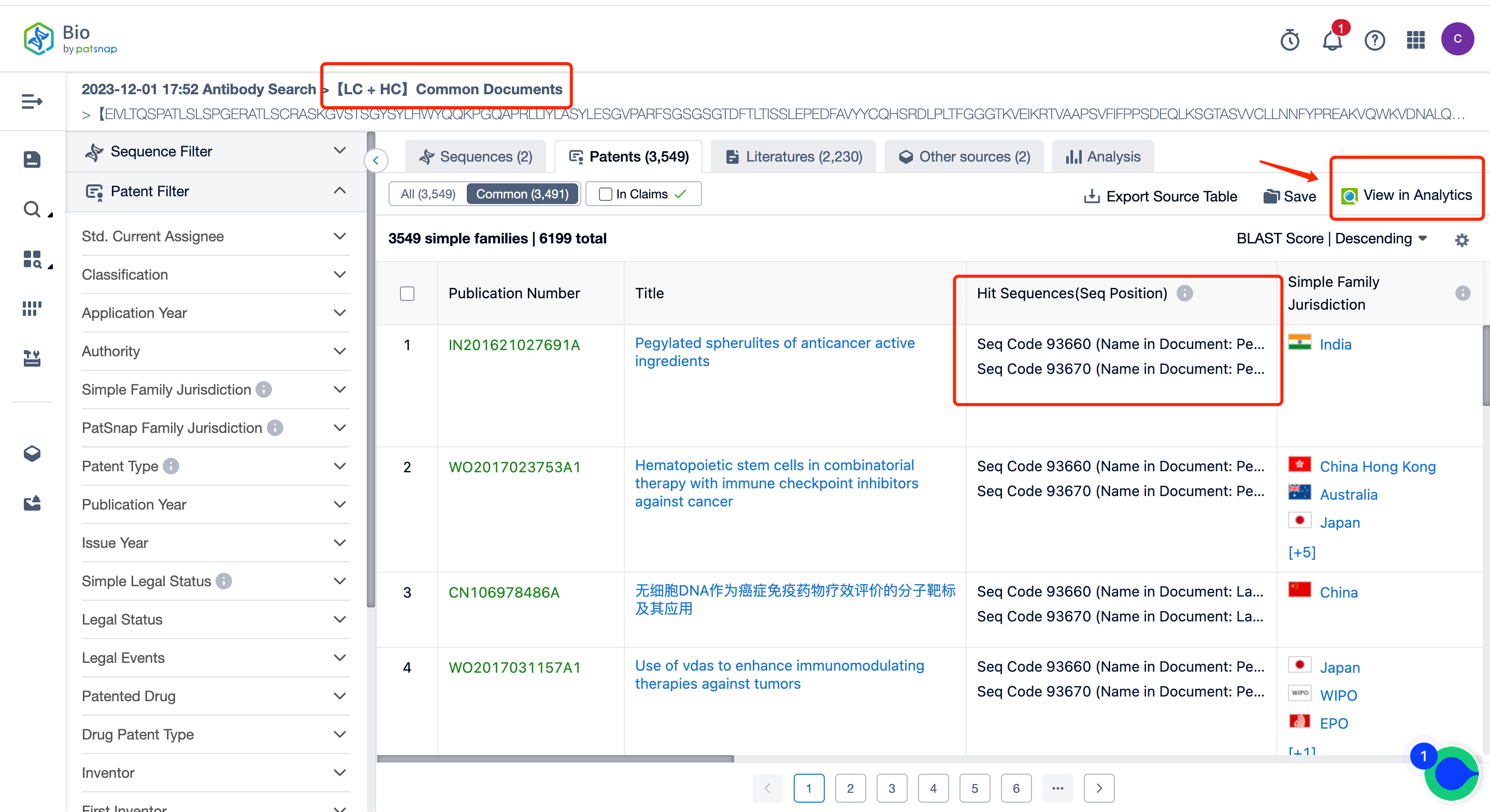
In addition to various sequence combination analyses in the report, it also includes common documents analyses. For example, you can see how many shared public patents there are for the heavy/light chain, what these patents are, and you can intuitively see the quantity and click to jump to the patent list page.
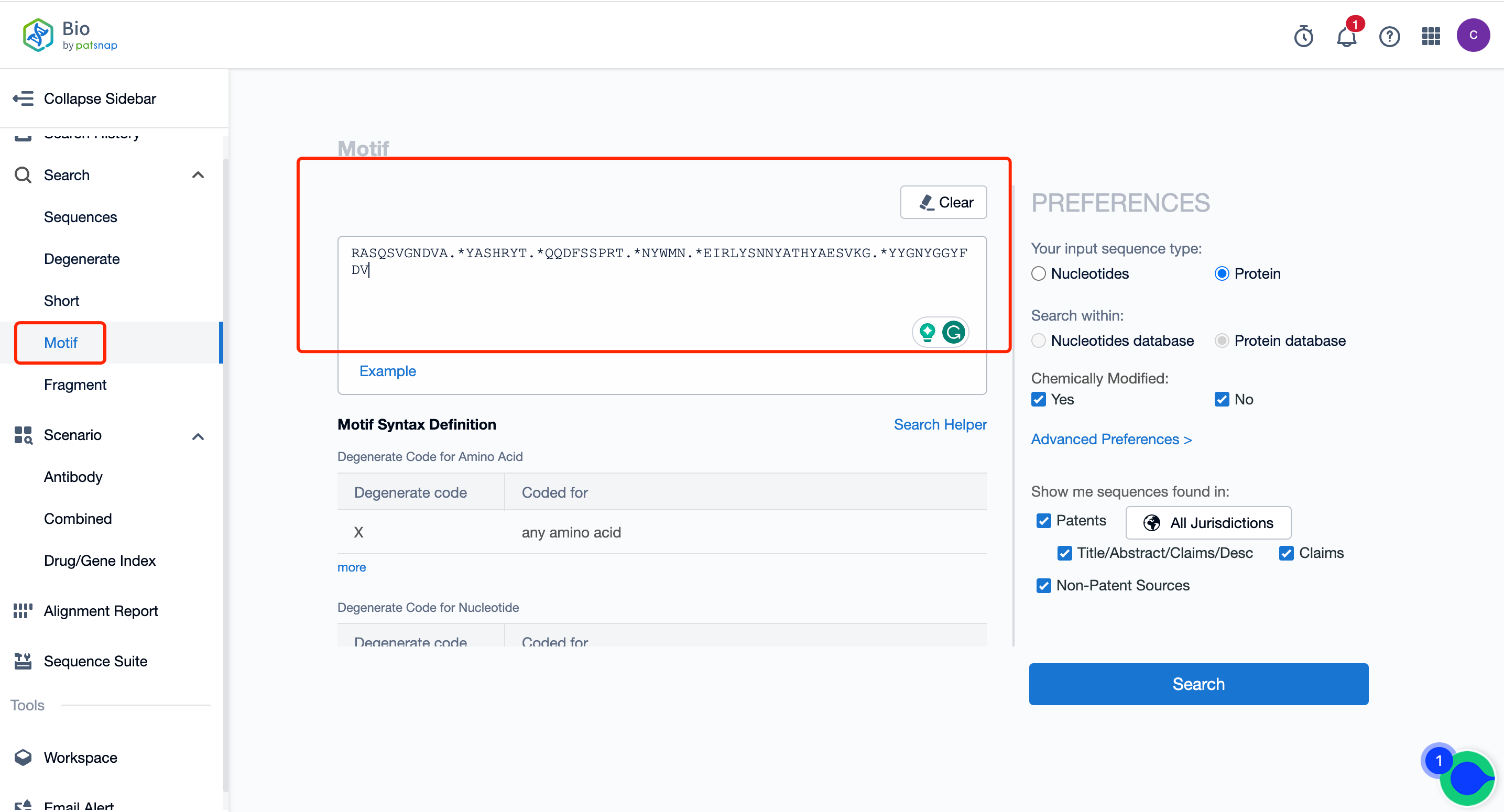
III. Motif search in the Bio database can be used to retrieve scFv sequences based on CDRs.
Some cell therapy researchers are interested in scFv sequences, and we can use the Motif search function for retrieval.
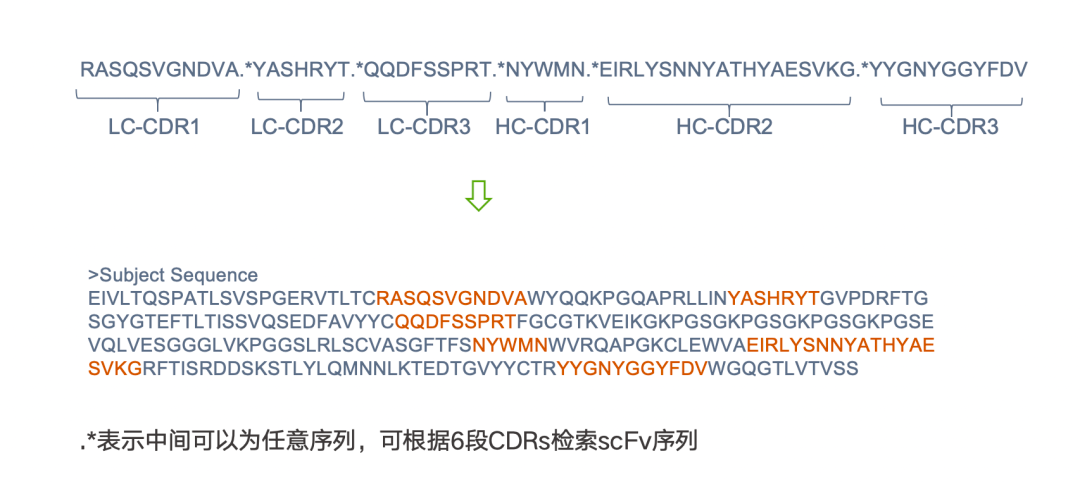
1)If the 6 CDR sequences are known, the Motif search can be used to retrieve scFv sequences containing these 6 CDRs. The specific operation and search formula are shown in the figure below: .* indicates that any sequence can be in the middle, with any sequence in between each CDR; this can assist us in quickly identifying all sequences containing these 6 CDRs.
2)If the CDR sequences are not definite, wildcards can be used to define possible CDRs, with amino acid mutations set to match all sequences that meet the conditions in the entire sequence library.
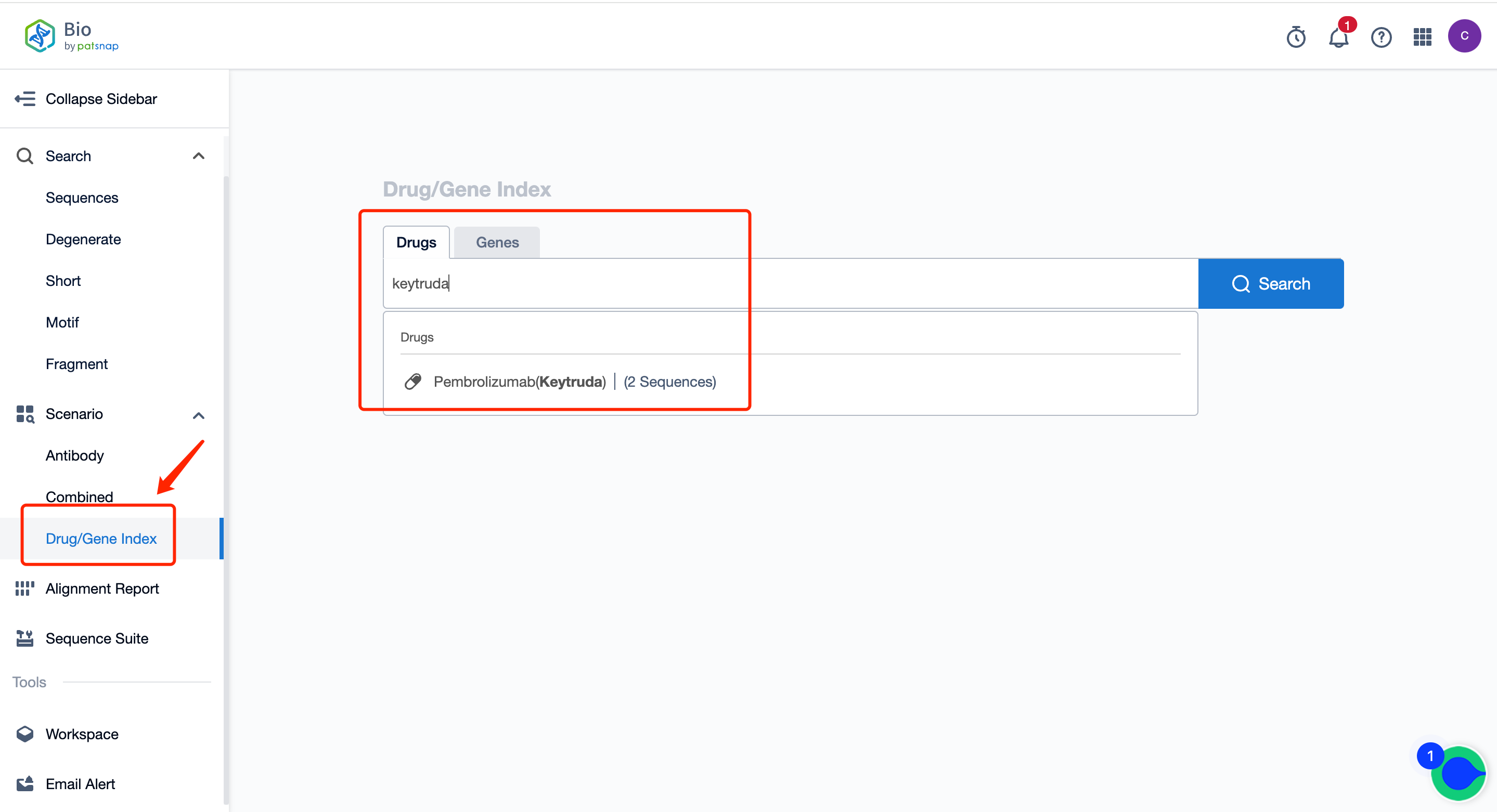
IV. The Bio database's Drug/Gene Index search can be used to retrieve antibody light and heavy chain sequences, quickly querying their patent and public literature information.
Simply search for sequences using drug/target names, such as Pembrolizumab light and heavy chains, PD-1 related antibody sequences… The specific operation steps are as follows:
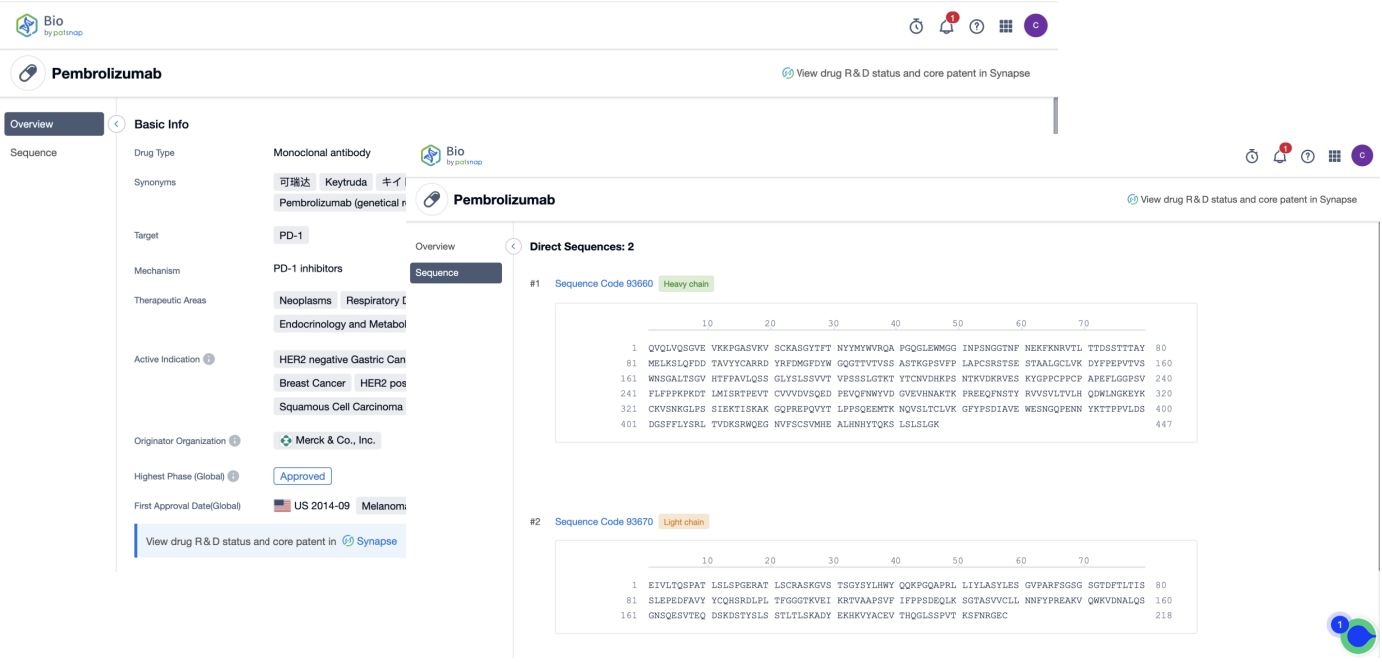
First, register for a free Patsnap Bio sequence database account, and enter the home page scene's Drug/Gene Index section. Simply enter the antibody drug name that you want to query. The system will then show the drug type, target, mechanism of action, clinical analysis, and some other basic information for this drug. The drug's light and heavy chains can also be viewed.
Similarly, switch to the genes search, and directly enter the name of the target you want to query. The system will display all sequences manually tagged related to this target. On the sequence list page, the search can be narrowed down based on antibody sequence, associated drug names, etc. The sequence details, as well as all of its related patents, literature, and open-source data, can also be viewed on the results page. Here you can select similar types of antibody sequences and directly save them to your workspace for online sequence comparison.
◆ In the following article, we will explain the sequence search method for CAR expression frames.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.